Title: Continuous in situ generation and reaction of phosgene in a microflow system
Authors: Shinichiro Fuse, Nobutake Tanabe, and Takashi Takahashi*
Journal: Chemical Communications
Affiliation: Department of Applied Chemistry, Tokyo Institute of Technology, Tokyo, Japan.
Take-Home Importance according to the Authors: Continuous in situ generation of phosgene and its use in acid chloride formation in a microflow system were demonstrated. The acid chloride was subsequently coupled with an amine in high yield without severe epimerization.
Take-Home Importance according to the Blogger: How to safely use a toxic gas on a potentially commercialized setup.
Summary: Acid chlorides are highly reactive carbonyl derivatives that can be very useful in generating reactive precursors for the subsequent synthesis. Phosgene, COCl2, is one of the simplest acid chlorides with chlorides as both substituents. So naturally, if one chloride reacts, there will still be a reactive acid chloride left. This process can be useful to activate a more thermodynamically stable carboxylic acid, by producing CO2 and HCl. Also, the carboxylic acid derivatives can be sometimes commercially available and less costly compared to the acid chloride derivatives. Since phosgene can be produced from CO and Cl2, its cost should be rather reasonable.
However, there are MANY things that must be considered before using phosgene as a reagent. First, phosgene should never be used as a reagent intentionally as it is. It is used as a chemical warfare agent during World War I, so humanity had experience with its potency. If you can avoid using phosgene as a starting material, please do so.
Now, if phosgene cannot be substitute by any other means, diphosgene and triphosgene should be used instead because they are solid at room temperature, opposed to a toxic gas. Note that with even trace moisture, diphosgene and triphosgene will decompose and release phosgene. So make sure you had proper training and some experience in handling air-reactive materials and your laboratory equipments are up to the task. Still pay absolute attention when you are handling the material (the toxicity does not decrease, but the solids do extend the probability of you surviving the experiments). Of course, always work in small quantities and decrease exposure time.

Safety aside, there may be some chemical disadvantages. Since acid chlorides are reactive, the reactant may lose stereogenic center and become epimerized. Also, the side product HCl may cleave other functional groups such as t-butoxy. So work fast is the key to reduce the amount of side products.
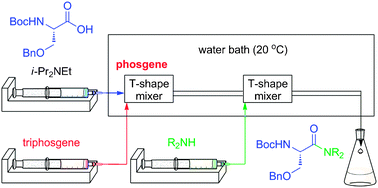
So what to do? Utilizing a flow-reactor, phosgene precursor can be generated in situ with minimal excess (5%). Since the reaction is done in microliter scale, If the amide is the desired product, immediate amidation, with various amines, will certainly decrease epimerization of the acid chloride. With optimized flow, the reaction can be completed in mere 20 seconds while suppressing generating the other isomer. the results are reproducible. Afterwards, mixture containing the product can be quenched with saturated NH4Cl (aq) in CH2Cl2. Although yield can be slightly lower compared to the batch synthesis, the selectivity is quite strong.
Chemistry-wise, there is nothing new. It could actually be one of the most straightforward pathways. But how can chemists improve their reaction systems? Maybe taking a peek at a more traditionally chemical engineering concept, and then magic happens.

azmanam
November 21, 2011We used thiophosgene (CSCl2) quite frequently in my grad school lab. One day, during lunchtime of course, someone broke a bottle of thiophosgene in our refrigerator room. it’s the only time I’ve ever seen a fire alarm pulled, and there was quite an emergency personnel presence on campus. It even made the campus newspaper. Everyone evacuated safely, even the person who dropped the bottle at her feet. Definitely not a fun chemical to work with.
Nice post, good work 🙂