Title: Thermoelectricity Enhanced Electrocatalysis
Authors: Tiva Sharifi, Xiang Zhang, Gelu Costin, Sadegh Yazdi, Cristiano F. Woellner, Yang Liu, Chandra Sekhar Tiwary, and Pulickel Ajayan
Publication Journal and Link: Nano Letters, DOI: 10.1021/acs.nanolett.7b04244
By: Jared Mondschein
By 2030, annual energy demand is expected to reach 30 Terawatts, about a 300% increase from current energy demand. The continued reliance on the burning of fossil fuels will result in the increased release of greenhouse gases and other noxious pollutants, a scenario that many think needs to be avoided. Inventing environmentally friendly, cost-effective and highly efficient clean energy technologies is therefore a critical scientific challenge.
One significant problem associated with renewable energy devices is the current lack of energy storage solutions. However, nature has already developed a clean and efficient method for the storage of renewable energy. In a process called photosynthesis, plants store solar energy in the form of chemical bonds by rearranging the bonds in water and carbon dioxide to form sugars. Plants can then use these sugars to power their growth. Photosynthesis requires a complex network of enzymes and light absorbers to function properly, and is considered by some to be much too complex to replicate in a device. But what if we strip out all of the biology and focus on the bare essentials of this process?
Turns out, if we rearrange the bonds in just water, we can make H2(g) and O2(g). H2(g) is a great energy carrier with a gravimetric energy density that exceeds that of fossil fuels, while O2(g) is a harmless byproduct. Water splitting can be performed electrochemically, wherein thermodynamic and kinetic barriers can be overcome with the application of a voltage. In this process, the oxidation of water at the anode produces electrons, oxygen gas, and protons. The protons and the electrons then combine at the cathode to produce hydrogen fuel. Any source of energy could be used to power this process, including wind power and solar power.
In this study, the researchers examined a set of materials that could use heat to power electrochemical water splitting. This class of materials is called thermoelectric materials, which produce a voltage when in contact with a temperature gradient. This voltage could then be used to power the production of hydrogen fuel from water. While the scientists applied the heat via a heater, it is rather easy to imagine a technology that utilizes heat given off by inefficient devices such as engines, large machinery, or even ambient heat! Such an idea had never been tried before.
As a proof of concept, two thermoelectric materials were synthesized: Sb2Te3 and Bi0.5Sb0.5Te3. Their hydrothermal synthesis resulted in hexagonal nanosheets with lateral dimensions of approximately 1 micrometer. The successful synthesis was confirmed via a host of materials characterization techniques, including x-ray diffraction, high-resolution transmission electron microscopy, energy dispersive spectroscopy, and electron diffraction.
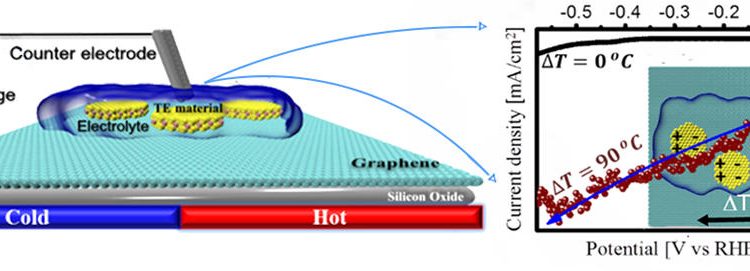
The scientists then applied a temperature gradient to these materials and studied their ability to serve as the cathode (where protons are reduced) in a water-splitting device. While the activity of Sb2Te3 towards proton reduction didn’t change due to the applied heat, Bi0.5Sb1.5Te3 became much more active. The optimal temperature gradient was determined to be 90 °C. Stability experiments were then utilized to determine that Bi0.5Sb1.5Te3 could reduce protons to hydrogen with an applied temperature of 90 °C for >225 minutes (Figure 1).

Figure 1. (a) The application of a 90 °C temperature gradient increased the current densities that could be produced by Bi0.5Sb1.5Te3, drastically improving its ability to reduce protons to hydrogen. (b) Stability experiments indicated that Bi0.5Sb1.5Te3 could produce device-relevant current densities, and therefore reduce protons, for prolonged time periods with a 90 °C temperature gradient.
The idea of storing energy from heat in the form of chemical bonds is fascinating. In this proof-of-concept study, the researchers provide sufficient data to indicate that a functioning device may be within sight. The capability to store heat energy in chemical bonds like the H-H bond in hydrogen gas could be a game changer. Hydrogen gas can already be transported and used in a similar fashion as gasoline. Today, over twelve thousand forklifts currently being powered by hydrogen fuel and >20 hydrogen refueling stations in California for refueling hydrogen-powered cars. An environmentally friendly and highly efficient method of turning waste heat into hydrogen fuel may enable widespread global adoption of these technologies and a shift away from burning fossil fuels.