Fluffy yet crunchy puffs coated in butter and salt, popcorn is undisputedly an ideal snack. Plus, listening to a handful of corn kernels pop with wild abandon is almost therapeutic. In my home, popcorn is so well loved it has its own ridiculous nickname: “p-corn.” But just as a perfect bowl of popcorn is the best, burnt popcorn is the absolute worst – in taste, texture, and most of all, smell.
However, in the Cao lab at Yangzou University, China, the smell of burnt popcorn is no accident. It is a happy reminder of their recent discovery that popcorn can be converted into a honeycomb made of porous carbon flakes, ideal for use in a supercapacitor.

Supercapacitors are basically fancy high-power batteries that are stable, long-lived, and perfect for electric cars. Initial tests of supercapacitors made with the burnt-popcorn honeycomb are promising: Jianhua Hou and co-authors made devices with the highest energy output of any biomass-derived carbon material to date.
As reported in Chemical & Engineering News, it all started when Hou wondered about the microscopic structure of popcorn while eating the snack with his daughter – and that simple question led to heaps of inedible, blackened popcorn. And a very exciting new material.
Supercapacitors are composed of two main parts: a solid “electrode” submerged in a liquid “electrolyte.” Just like a sports drink, the electrolyte contains electrically charged molecules called ions. The supercapacitor works by separating the positive and negative ions to different sides of the device, where they are stored on the solid electrode. In this case, that electrode material is made from popcorn.
The goal of any supercapacitor is to store as many ions as possible. So an ideal electrode must have plenty of space for ions to call their home. In other words, the electrode must have a high surface area, and is therefore composed of “nanopores.” Much like the pores of your skin, nanopores are tiny holes arrayed inside a larger structure, but are about 100,000 times smaller. These small holes dramatically increase the surface area of the structure, and can be optimized in size, shape, and chemical composition. This makes it very tricky to design materials with nanopores that are just right, and researchers have developed sophisticated methods to create new nanostructured materials.
But, until now, no one thought popcorn could be the solution.
The build-up of pressure inside a corn kernel that leads to a superfast expansion of the kernel’s inside is known scientifically as the “puffing effect.” The volume of the corn is multiplied 20 times in the puffing process, while the mass doesn’t change much – suggesting a highly porous structure.
Hou answered his question about popcorn’s microstructure when an electron microscope revealed to him a highly regular honeycomb flake-like structure (Figure 1a-b). Using the already porous popcorn as a template, Hou and co-authors had the brilliant idea to burn the popcorn in microwave for 8 minutes to convert it to pure carbon. They then “activated” the material with an application of potassium hydroxide (KOH), a method that has been used on other carbon-based supercapacitor materials.
“We suggest using a fume hood,” Hou told Chemical & Engineering News, as the smell of burnt popcorn does not garner much goodwill hanging around your lab bench all day.
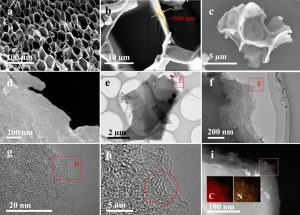
The final result is a remarkably elaborate carbon nanostructure with a honeycomb microstructure chock-full of nanopores, depicted in high definition with electron microscopy in Figure 1. These images show that the original honeycomb flakes are preserved (Figure 1c) and that the application of KOH actually etches into the ~500 nm thick flakes, creating thin layers stacked on one another (Figure 1d-f). By zooming in even farther, the researchers found that these layers are composed of nanopores, which look a bit like a pile of worms in Figure 1g-h.
The resolution of this electron microscope is so good that these nanopores can be directly measured – and they come in just under one nanometer across. That is super tiny, and super perfect for ion storage in a supercapacitor.
The researchers made several different samples of their popcorn-derived carbon flakes (PCFs) with some variation in pore size and surface area. When made into a supercapacitor with an ionic liquid electrolyte and run through a number of standard device performance tests, they found that larger surface area PCFs yield a better supercapacitor (higher capacitance, higher energy density, and higher power).
The thin, stacked layers of micropores revealed by electron microscopy also reduced the “ion diffusion distance” in PCFs compared to other materials, which allowed ions to move more easily through the material. Combined with the high surface area and < 1 nm pore size, this resulted in a record-setting material. And it’s all thanks to popcorn!
Despite major olfactory drawbacks, converting popcorn to a supercapacitor electrode could be well worth it. Corn is abundant and renewable, and burning it up in an industrial-sized microwave is cheap, energy efficient, and easy to scale up.
While today the smell of burnt popcorn sends me sprinting to the kitchen, perhaps tomorrow it will bring to mind the peaceful image of an affordable, environmentally friendly electric car.
Paper title: Popcorn-Derived Porous Carbon Flakes with an Ultrahigh Specific Surface Area for Superior Performance Supercapacitors
Authors: Jianhua Hou, Kun Jiang, Rui Wei, Muhammad Tahir, Xiaoge Wu, Ming Shen, Xiaozhi Wang,
and Chuanbao Cao
Year: 2017
Journal: ACS Applied Materials & Interfaces
Article link: pubs.acs.org/doi/abs/10.1021/acsami.7b07746

