Title: A synthetic polymer system with repeatable chemical recyclability.
Authors: Zhu, Jian-Bo, Eli M. Watson, Jing Tang, and Eugene Y-X. Chen.
Journal and year: Science, 2018
PLASTICS? I know what you are thinking- HOW CAN PLASTICS SAVE THE EARTH? Aren’t they doing just the opposite? – Yes, you are right! Plastics are harming the environment. Our current living habits clearly guarantee that we cannot live without plastics and our current plastics disposal habits guarantee that the earth will not survive this human-made disaster. We have been circling the argument of plastic vs planet for decades now. So, how might plastics actually help save our planet and ensure sustainable living?
If plastics are infinitely recyclable, it is possible to strike a balance between plastic usage and planet health. You might be thinking, we already have recyclable plastics. However, you will be surprised to know that only 5% of the plastics ever manufactured have been recycled. Recycled plastics lose a part of their functionality in recycling and are therefore manufactured as lower value secondary products, which are not recyclable. These plastics, in addition to the 95% of plastic that is never recycled, end up in landfills or oceans, polluting the environment and creating need for more plastic generation. Thus, the cycle continues…
Imagine if the plastics manufactured were recyclable not just for a few cycles, but infinitely recyclable without losing their functionality. Then, we could prevent plastic disposal into the environment and also curb the need for additional plastic generation. We all could be operating from a fixed pool of plastic for decades.
But does infinitely recyclable plastic exist in reality? Can we synthesize this “magical plastic?”
There is a central paradox to the recyclable plastic problem. The plastics that are easily recyclable and can be converted into new plastic do not have robust mechanical properties needed for several applications of plastics. And the plastics that have phenomenal mechanical properties are not easily recyclable for multiple cycles. So, the challenge essentially boils down to synthetic chemists combining recyclability and robustness in one kind of plastic.
In the article we are discussing today, Zhu et al. synthesized a new kind of repeatedly recyclable plastic which retains the required properties even after infinite recycling.
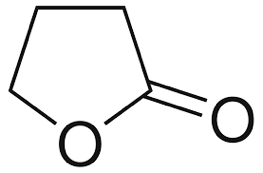
Before diving into the design aspects of plastics, let’s learn a few basics. Plastics are polymers, which are molecular structures consisting of a large number of similar units called monomers. For good mechanical properties in plastics, the polymers need to be semi-crystalline or crystalline. However, a higher degree of crystallinity makes it harder to recycle without losing its properties.
To design the “magical plastic”, Zhu et al. started with g-butyrolactone (GBL) which has a highly stable five-membered ring as shown in Figure 1. A cyclohexyl ring (6-membered carbon ring) was fused to the GBL ring to form a monomer unit. The fused cyclohexyl ring can have two possible positions in 3D space with respect to the GBL. This study of 3D arrangement of atoms and molecules is called stereochemistry. Depending on the orientation of the cyclohexyl ring, two different monomer units are generated as shown in Figure 2.
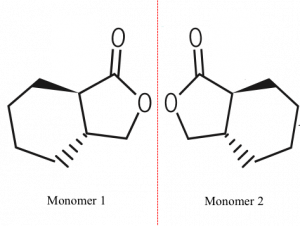
These monomer units were separately combined to form a polymer. The steric hindrance and stereochemistry of the ring monomer makes the two polymers semi-crystalline with thermal and mechanical stability. The ring structure provides both robustness and recyclability.
In addition, the two monomers are mirror images of each other, just like our right and left hand. Such mirror imaged chemical structures are called enantiomers. These enantiomeric monomers give rise to enantiomeric polymers. Mixing these enantiomeric polymers gave a new polymer which had more thermal stability, thereby expanding the use of these polymers in high temperature applications like hot coffee cups.
Figure 3 shows the three polymers made by Zhu et al that have infinite recyclability and robust mechanical and thermal properties. Soon these polymers will be industrially manufactured as plastics, starting a new era where plastics help save the planet.
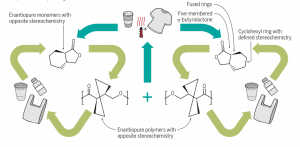
Synthetic chemistry has opened pathways to make plastics a component of sustainable environments. A unique approach of combining stereoselectivity in ring isomers has allowed the synthesis of polymers with high mechanical and thermal strength, in addition to recyclability. With this design of repeatedly recyclable plastics, we minimize the generation of plastic waste and of plastic derived from petroleum resources. Thus, we are saving our planet Earth.

