Michelle Ehrenberger
Title: “Cationic Silver Nanoclusters as Potent Antimicrobials against Multidrug-Resistant Bacteria”
Authors: Zil-e Huma, Akash Gupta, Ibrahim Javed, Riddha Das, Syed Zajif Hussain, Shazia Mumtaz, Irshad Hussain, Vincent M. Rotello
Journal: ACS Omega
Year: 2018
https://pubs.acs.org/doi/10.1021/acsomega.8b02438
Featured image and figures used with permission via ACS AuthorChoice open access
Multidrug resistant (MDR) bacteria, or “superbugs”, such as methicillin-resistant Staphylococcus aureus (MRSA) have become a huge problem all over the world. Resistance usually occurs over time through natural selection, when some of the infecting bacteria coincidentally have a genetic mutation that makes the drug less effective against them [1]. While all the other infecting bacteria may die, these resistant bacteria are likely to live on, replicate, and even infect other people [1]. If different drugs are then used to treat the now-resistant strain of bacteria, multidrug-resistance can develop over time, especially when antibiotics are prescribed when they aren’t needed [2], or when a person takes a subtherapeutic level of the drug (meaning: less drug than experiments show are required to fully wipe out an infection [1]). Because of this, in the United States, an infection contracted in a hospital—where a high number of antibiotics are prescribed [1]—has a 60% chance of being multidrug-resistant. The problem is even greater in developing nations where antibiotics are often available without a prescription [1].
New antibiotics effective against MDR bacteria are desperately needed. One solution is simply to make as many different antibiotics is possible, hoping to find at least one that can wipe out an MDR infection. However, resistance could also develop to these drugs, so a better strategy would be to find a drug that kills MDR bacteria through a mechanism that doesn’t put selective pressure on a mutated gene to proliferate. In one research lab, Huma et al. believe they may have found this method.
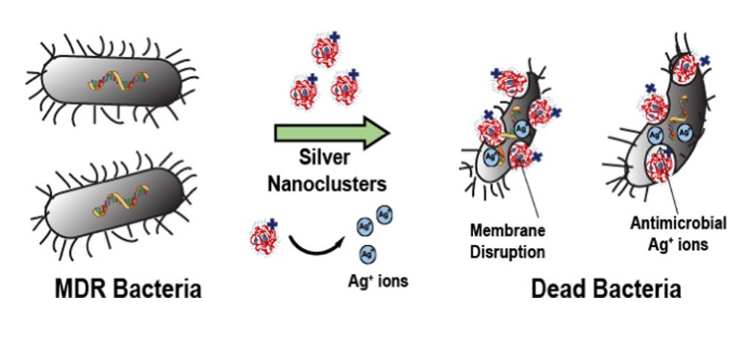
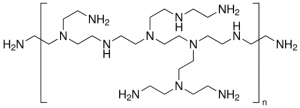
The scientists combined two different materials known to damage bacterial cell membranes: a cationic (positively charged) polymer called “branched polyethylenimine” (bPEI) and cationic silver ions (Ag+), which are also known to disrupt bacterial ribosomes and enzymes [3]. When silver nitrate (AgNO3) is dropped into a buffer solution containing bPEI, brought to pH 7, and combined with a small amount of formaldehyde, in 24 hours small structures called bPEI-Ag nanoclusters (NCs) are formed. These nanoclusters are tiny balls of polymer and silver ions that are an average of only 3nm in diameter. A solution of bPEI-Ag NCs looks yellow and—when UV light is applied—emits blue fluorescent light. Once the nanoclusters were obtained, the scientists tested their ability to kill 12 pathogenic bacteria, including MRSA, that cause urinary tract infections and one nonpathogenic bacteria as a control.
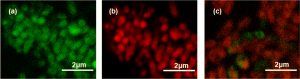
The scientists used a few different tests to determine the “minimum inhibitory concentration” (MIC) of the bPEI-Ag NCs, meaning the lowest concentration needed to stop bacteria from growing. First, they simply measured the optical density of bacterial suspensions in water (how opaque a suspension is due to living bacteria floating around in it) before and after incubating with bPEI-Ag NCs. In another experiment, they applied both a green fluorescent stain which can only enter living cells and a red fluorescent stain which can only enter dead cells, and viewed the fluorescence via confocal microscopy. Their results were consistent: growth of all tested bacterial strains was inhibited at a much lower concentration by bPEI-Ag NCs (0.25-0.015nM) than either bPEI (32nM) or silver nitrate (2-0.125nM) alone.
The scientists believed that the bPEI-Ag NCs were so effective for a few reasons. Firstly, the positive charges on both bPEI and Ag+ should be able to disrupt the negatively charged bacterial cell membrane. They supported this idea by looking at bPEI-Ag NC-treated bacteria under an electron microscope, where they did see damaged-looking membranes. Secondly, the using both bPEI and Ag+ together in one cluster should lead to increased membrane perforation in addition to silver’s intracellular enzyme disruption. And thirdly, since the silver ions are tangled in the bPEI polymer until the two interact with a bacterial membrane, this leads to a longer and slower release of Ag+ onto the membrane than using AgNO3 does, keeping the drug around for longer. All of these factors make bPEI-Ag NCs very promising.

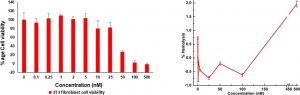
However, one vital set of tests remained: would the bPEI-Ag NCs be toxic to human cells? Since mammalian cell membranes are more stable and less negatively charged than bacterial cells, the researchers were hopeful that their nanoclusters would not be able to puncture human cells. First, they tested the ability of bPEI-Ag NCs to kill fibroblasts—the cells in all the kinds of connective tissue throughout the mammalian body. The IC50 (the concentration required to kill 50% of tested cells) of bPEI-Ag NCs against fibroblasts was 38nM, which is considered to be safe for an antibiotic. Then, they tested the ability of bPEI-Ag NCs to lyse (break open) human red blood cells, since these are particularly susceptible to lysis by nanocluster drugs. The HC50 (the concentration required to lyse 50% of tested cells) was over 500nm. Thus, the developers of bPEI-Ag NCs had their first evidence that the drug may be safe enough to be used to treat infections in humans.
There is still a long way to go, however, with years of clinical trials likely required before the drug could end up in a pharmacy. Still, the future is promising for bPEI-Ag NCs, which can kill a wide array of pathogenic and even multidrug-resistant bacteria, without being too toxic to human cells studied thus far. So if anyone ever asks how to make silver more valuable, the answer isn’t adding gold, platinum, or diamonds, but a cationic polymer named branched polyethylenimine!
[1] Levy, S. B.; Marshall, B., Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine 2004, 10, S122.
[2] Wenzel, R.P., Edmond, M.B. Managing antibiotic resistance. New England Journal of Medicine, 2000, 343, 1961-1963.
[3] Rai, M.K., Deshmukh, S.D., Ingle, A.P., and Gade, A.K. Silver nanoparticles: the powerful nanoweapon against multidrug‐resistant bacteria. Journal of Applied Microbiology, 2012, 112, 841-852.