Title: Trojan Antibiotics: New Weapons for Fighting Against Drug Resistance
Authors: Xuejiao Wang†⊥, Yuxin Liu‡⊥, Yiyang Lin†, Yuchun Han§ , Jianbin Huang†, Jing Zhou‡ , and Yun Yan*†
Journal: ACS Appl. Bio Mater.
Our modern society irreversibly relies on antibiotics to fight against disease, treat sewage, and keep our livestock alive. Although their impacts are intended to be positive, accumulation of these antibiotic molecules in the environment has resulted in microbial resistance, where bacteria have mutated and evolved to resist conventional antibiotics. Long story short, bacteria are getting stronger and our line of defenses has weakened significantly in recent years. Therefore, scientists today are tirelessly searching for unconventional methods to battle antimicrobial resistance. A variety of approaches exist and today’s Chembite focuses on promising developments with antibiotics using “trojan horse” tactics!
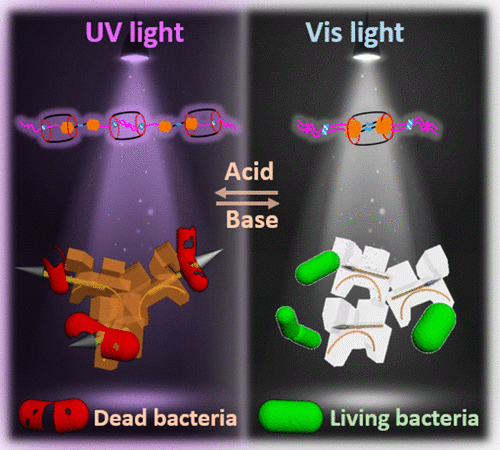
The story of the Trojan Horse comes from Greek mythology written during the Trojan War. After a lengthy and ineffective siege on the independent city of Troy, the Greek soldiers developed a clever plan to give the city of Troy a “gift” to signify defeat in the shape of a giant wooden horse. Instead of a gift, the giant wooden horse was filled with Greek soldiers. The people of Troy fell for the ruse and the soldiers snuck out at night to let in the rest of the army, and thus the Greek army successfully completed their siege and won the war. Similarly, these researchers develop a “switchable” Trojan horse antibiotic that appears harmless to the bacteria under normal conditions. Yet, when the pH is changed or UV light is shone, the antibiotic is released and successfully deters the bacteria. Essentially, you cannot pass the enemy’s defenses unharmed if they know you are an enemy.
So how does the disguise work? The antibiotic itself is a supramolecular structure (Figure 1), composed of a long string-like molecule (A2G) and a barrel-like molecule (CB[7]). The surface is decorated with sugar-like molecules that are very similar to the outer surface of the bacteria (wall or membrane). Thus, the antibiotic is disguised as a friend to the bacteria. Even more, the antibiotic has switchable potency depending on what conditions the bacteria are exposed to. The molecule is designed to be harmless at a weak basic pH around 9 and under ambient light. At pH 4 and UV irradiation (Figure 1), the poisonous azobenzene part of the structure (blue rectangle in Figure 1) is exposed and the antibiotic reaches full potency. The sudden change in pH or UV radiation catch the bacteria by surprise so that it doesn’t have time to develop a resistance to the antibiotic which is pivotal when scientists are designing new antibiotics.
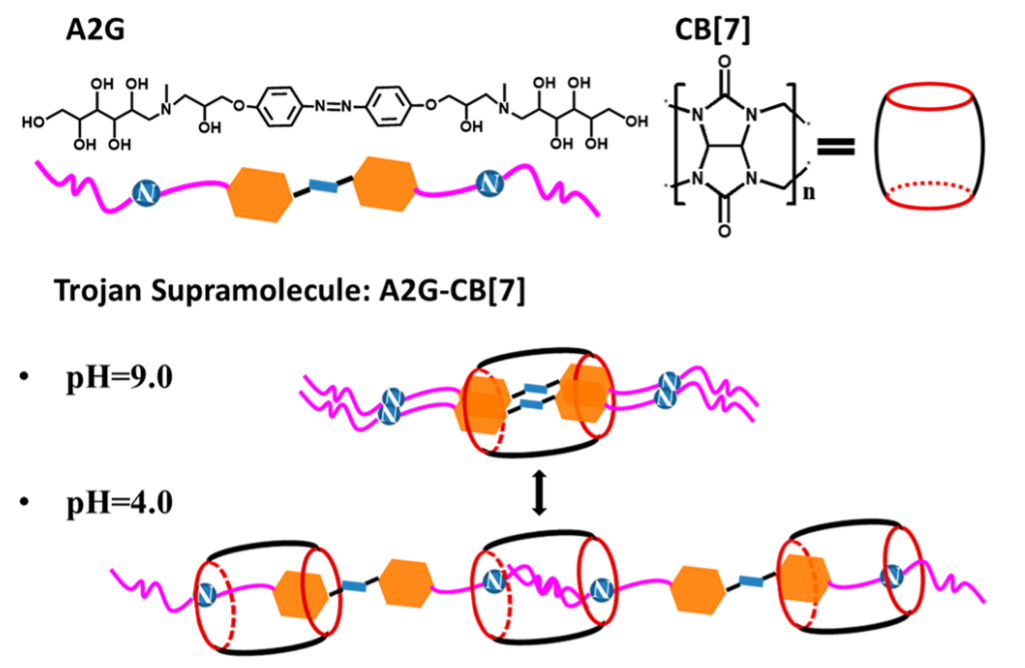
The bactericide is up to 10 times more effective than conventional drugs because a 10x smaller dose can show the same effect. Visual evidence of this is shown in Figure 2, where the bacteria E. coli are exposed to the Trojan antibiotic at varying conditions. Bacteria that are alive are green while the dead are shown in red. The researchers also show that the UV source can be removed, and the pH reversed back to normal to prevent the build-up of active antibiotic. This stops bacteria to replicate or evolve to survive the toxic molecule. Therefore, switchable antibiotics can prevent this time lapse of exposure and thus prevent the bacteria from developing resistance.
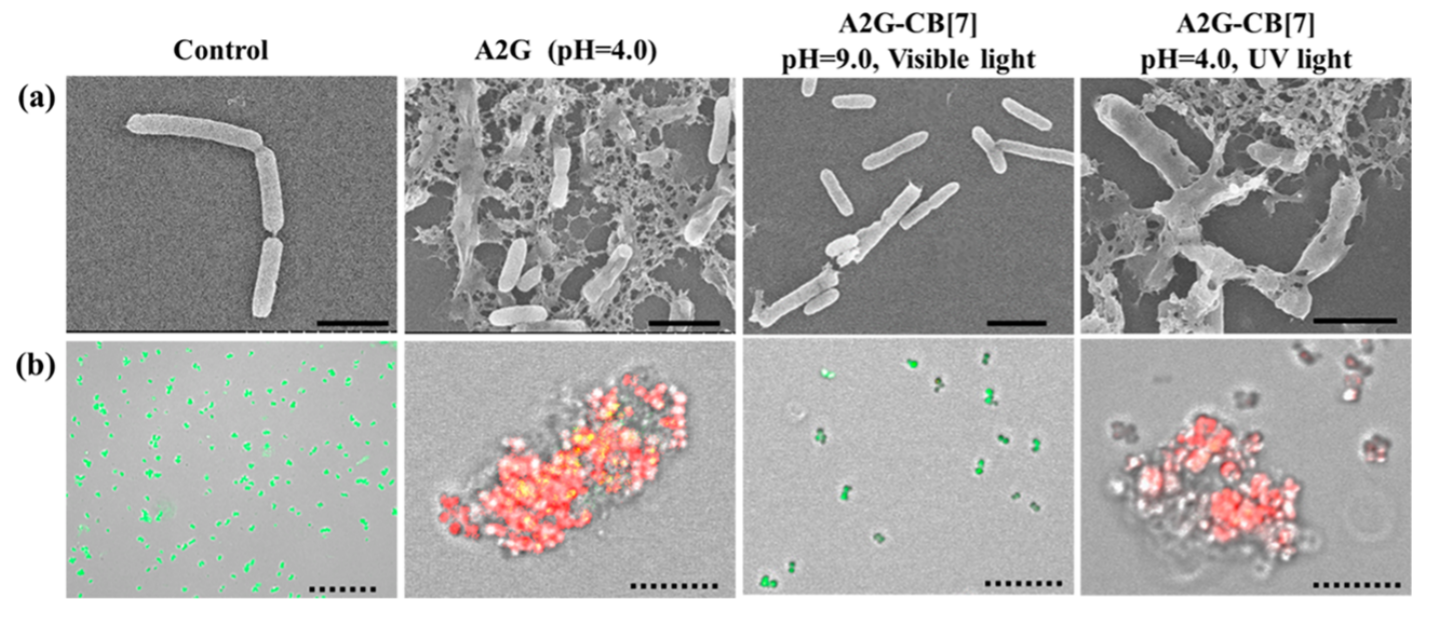
In conclusion, the researchers have developed a method to make antibiotics more effective by tricking the bacteria into thinking the molecule is harmless, then activating a death switch. Further investigation with other antibiotics and more dangerous strains of bacteria like MRSA could lead to a switchable system that can work in a wider variety of scenarios.