Authors: Geoffrey Rivers, Li Yu, and Boxin Zhao
Year: 2019
Journal: Langmuir
Featured image: Adapted with permission from (Langmuir 2019, 35, 48, 15897-15903). Copyright (2019) American Chemical Society.
—
Wouldn’t you love to have a phone screen that didn’t crack when you dropped it? A t-shirt that generated electricity? Or maybe an advanced medical device that fits comfortably on your skin to monitor your health? All these possibilities rely on our ability to make electronic devices that are flexible; materials that can bend or stretch while still conducting electricity. Flexible electronics have been a popular topic for years now, and researchers have discovered many creative ways to make them- check out other Chembites articles to learn more (1,2,3,4). Here, we will take a look at a research group from Waterloo, Canada, that has made a flexible, conductive surface comprised of two very different nanomaterials working in tandem.
A preferred method to make a conductive surface is to print a water-based ink containing conductive nanoparticles onto a flexible substrate (such as fabric, paper, rubber, etc.). But how do these inked substrates stay conductive upon being bent or stretched? To be conductive, the molecules of the ink must stay physically close enough to transfer electrons—but stretching causes particles to move apart. So, what to do? Well, one trick is to apply the nanoparticle ink while the substrate is already stretched, such that upon release the particles are forced to compress, or “buckle”, into an S-shape (Figure 1). Now, when the material is bent, it maintains its conductivity because the conductive particles are not moving farther apart from each other, they are simply uncurling.
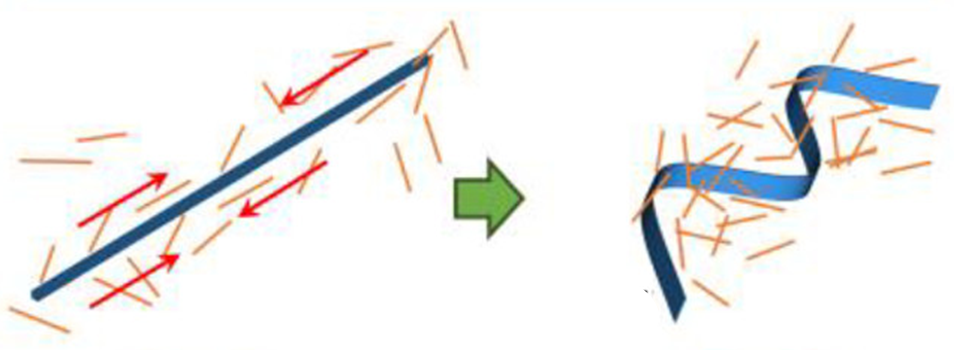
However, the fact that this method requires the substrate material to be pre-stretched before being inked can be a disadvantage. If the nanoparticles in the ink could be compressed before printing, that would increase the range of possible substrate materials and manufacturing methods.
While silver nanowires (one-dimensional particles) have been frequently studied as components for conductive inks, they are also notoriously unstable and can break down with use. Therefore, Rivers et. al. instead chose to investigate the use of two-dimensional silver nanobelts (AgNBs), which have the advantages of better thermal stability and a less-demanding synthesis. Still, there is a valid reason why AgNBs have not been previously considered—namely, that they are not water-soluble. While the particles could be made soluble by capping them with appropriately polar ligands, such capping molecules would also likely decrease the nanoparticles’ conductive ability.
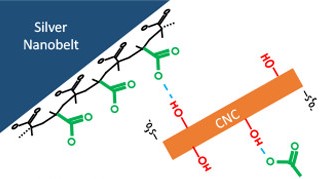
Rivers et. al. instead chose to combine their AgNBs with other, even smaller nanoparticles of biological origin which are water soluble: cellulose nanocrystals (CNCs), which have been shown to help various particles disperse in water more readily. In this case, the sulfates on the CNC surfaces can hydrogen bond with the methacrylic acids which cap the AgNBs (Figure 2). The CNCs then act as spacers, surrounding the AgNBs and keeping them suspended in water, forming a hydrogel. Gel-like properties are in fact desirable for many conductive inks, as they make printing easier to control.
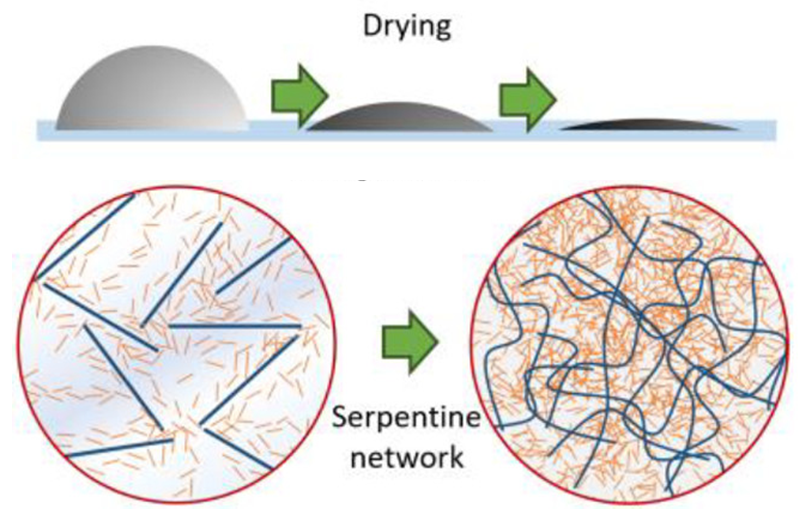
But we’re not done! Remember, we want not only a conductive ink, but one we can print on any flexible surface. For that, the AgNBs need to “buckle” and form an S-shape, but we want them to do so before printing them onto our desired substrate. Well, Rivers et. al. designed their AgNB-CNC gel to do exactly that, using an absurdly simple method: drying! When water is removed from the gel, the CNCs must move closer together to fill in the space. This puts pressure on the AgNBs, causing them to be compressed and buckle as intended (Figure 3).
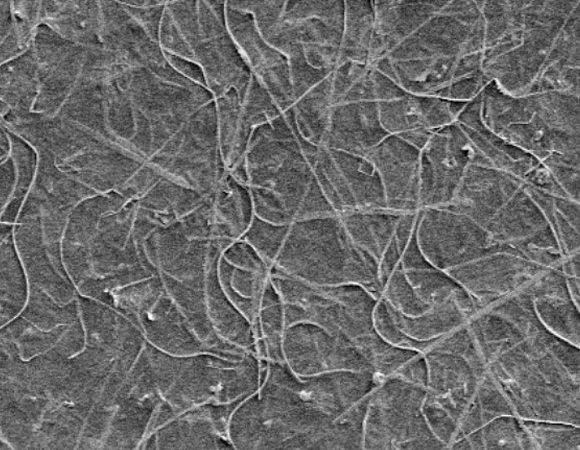
An important part of the work done by Rivers et. al. was to determine the right ratio of AgNBs to CNCs in the production of their ink. While CNCs are needed in order to disperse the AgNBs and form the gel at all, too many CNCs cause the gel to be too weak and simply fall apart. The researchers then printed the ink on strips of paper, and extensively tested how well the resistivity of the ink held up after being bent repeatedly. They found that the resistivity of the ink was virtually unchanged after 20 cycles of bending.
Overall, while many improvements to the ink stability, consistency, and drying rate need to be made in order to make this a viable option for manufacture, this study provides an interesting new combination of materials and methods for the further development of flexible electronics. Through manipulation of the physical and chemical properties of two existing nanoparticles, Rivers et. al. designed a new material with potential to overcome previous disadvantages. As a result, this study demonstrates just one of many ways in which materials chemistry can advance our technological capabilities.