Title: Solar eclipse demonstrating the importance of photochemistry in new particle formation
Authors: Tuija Jokinen, Jenni Kontkanen, Katrianne Lehtipalo, Hanna E. Manninen, Juho Aalto, Albert Porcar-Castell, Olga Garmash, Tuomo Nieminen, Mikael Ehn, Juha Kangasluoma, Heikki Junninen, Janne Levula, Jonathan Duplissy, Lauri R. Ahonen, Pekka Rantala, Liine Heikkinen, Chao Yan, Mikko Sipilä, Douglas R. Worsnop, Jaana Bäck, Tuukka Petäjä, Veli-Matti Kerminen & Markku Kulmala
Year: 2017
Journal: Scientific Reports 7
https://www.nature.com/articles/srep45707
August 21 was a big day in the US as many witnessed the spectacular total solar eclipse! The bright white sun was slowly covered by the moon, temperature dropped, sky darkened. As totality commenced, a sunset-like twilight surrounded us in all directions (imagine a 360° sunset). Then the process reversed: the sun shined again, temperature raised, and everything was back to normal.
We all know that it is a spectacular astronomical phenomenon. But that’s not all solar eclipses have to offer. They ‘shed light on’ chemistry as well! Indeed, they are the scarce occasions to study how sunlight influences particle formation in the atmosphere during the day. Ideally (but impractically), we would want to control the brightness of the sun and observe how particle compositions change. Solar eclipses provide a natural way to dim the sun while keeping other sky conditions fixed.
The bright clear sky is not as clear as it might seem. In fact, the Earth’s atmosphere is often covered by thousands of suspending particles, called aerosols. These particles could absorb and scatter sunlight, creating an opaque haze. These aerosols could be generated from sulphuric acid and nitrogen-containing organic compounds, which are the precursors of aerosol formation. The authors have used the 2015 partial solar eclipse in Finland to measure particle formation and deduce the role of these precursors in regulating atmospheric particle formation.
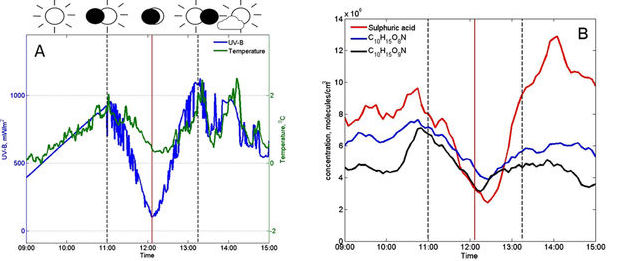
As the moon slowly covered the sun, there was a drop in UV light, which was accompanied by a significant drop in concentration of sulphuric acid and nitrogen-containing compounds (compare blue curve in Figure 1a and red curve in 1b). This therefore slowed down the particle formation process. Then as the sun was recovered after the eclipse, their concentrations increased and the particle formation was re-initiated.

Figure 1 (A) The measurements of UV-B radiation (mW/m2) and air temperature (°C), as well as (B) the concentration of aerosol precursor molecules on the eclipse day.
In particular, neutral particles with size 1-2 nm and 2-3 nm have shown a noticeable decrease during the eclipse (the black curves in figure 2). On the other hand, ions with size <2 nm were not affected much by the eclipse as they are mainly formed by ionizing radiation (the red and blue solid lines in figure 2). Although neutral particles with size 3-6 nm did not show a great decrease (the green solid lines in figure 2), their formation was suppressed compared to non-eclipse days as their concentration usually increased greatly around noon.

Figure 2 Time evolution of concentration of particles with different sizes. The vertical red line marks the time when the sun is blocked mostly by the moon; black lines mark the beginning and end time of the solar eclipse.
The authors here explored atmospheric chemistry during a solar eclipse and demonstrated connections between atmospheric photochemistry and aerosol particles formation. Perhaps, the solar eclipse not only teaches us chemistry, but also provides clues to air pollution and changing global climate in our atmosphere.

Pingback:What do solar eclipses teach us about Chemistry! – Eli's Science Blog