Featured Image credit: Charlie Crowe
With only the 1,000 (aka “ten hundred”) most commonly used words, our Chembiters explain science without the familiar comfort of the jargon only some people understand. The results can be funny, long-winded, or downright frustrating! Hang with us while we give this challenge a shot.
This week, Charlie Crowe explains his research making water droplets that simulate a cell-like environment. He starts with a “simplified” version using only the 1,000 most commonly used words, then re-writes an “unsimplified” version that relaxes the jargon rule.
Charlie Crowe: Making Things Like Your Cells!
Simplified
The inside of your cells is different than water. It is thicker and harder for things to move around. When we learn about how cells work, it makes sense to mirror what is actually in cells. To do this, we add other things to water to make it thicker.
This can lead to other cool things! Think about when you add something to water and it groups into a top and bottom part, like dressing (the food topping). This can happen if you just have water, too! If you add one thing that doesn’t like water and another that does, they will make two different parts. One part has the thing that likes water and the other has the thing that does not. Cells do this to group things together.
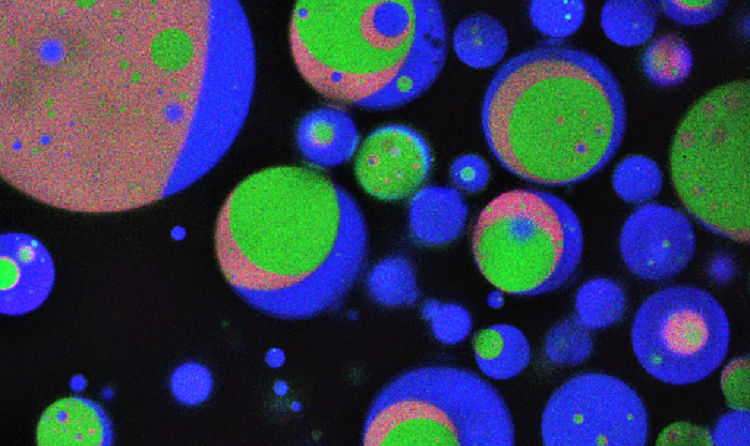
I make drops of water that have different parts inside of them. Then I put other things inside of the drops that can change with each other. Because things go to parts of the drops in different ways, I can control where and how fast these things change.
These drops can be made many different ways. You can shake them, but this makes drops that are all different. To make drops all the same, I push water through tiny areas so it breaks up into many small drops. If I control how hard the water is pushed, I can control what the drops look like. This makes all the drops the same.
I use this way of making drops to control what the inside of the drops are like. Then the drops can be made like the inside of cells and all be the same. Now I know the interesting things I see in the drops are because of the inside of the drops and not because they are all different.
Less Simplified
When biological reactions happen in your cells, they aren’t in solutions like water. Instead, there are many proteins and other molecules dissolved into the cytoplasm, a water-based fluid that fills cells, making it viscous and non-ideal. This is called “crowding.” So, when we study these reactions outside of cells, we use crowded solutions that simulate conditions inside cells.
By controlling what we add to the solutions, other interesting things can happen too. Just like oil and water form two separate phases, we can separate two water-based phases! Adding certain combinations of polymers causes the mixture to separate into two phases, each rich in one polymer. The cell already does this with different proteins and polynucleotides. This can increase the local concentration of certain molecules and keep them separate from other molecules.
My research explores this by making emulsion droplets with multiple phases inside of them. Because of this, different reagents are concentrated in different areas, which impacts how the reaction takes place.

These droplets can be made by mixing the water-based phase together with an oil phase. But these droplets are very different from one another, making it difficult to compare experiments — each droplet has a different environment, making reactions inside each one different. Instead, I use microfluidics, where the liquids flow through tiny channels. Then the droplets can pinch off (or “drip”) into the oil. By controlling how fast the liquids flow, I can make droplets that are all the same.
Microfluidics lets me be sure that all the droplets in an experiment are the same. This way, when I add crowding agents to mimic cells, I can be sure of the environment. Then I know that the effects on the reactions are because of the crowding, not because of differences in the droplets.
By Charlie Crowe
Thanks for reading Chembites’ Ten Hundred Word Challenge series! We don’t yet have another post lined up for next week, but stay tuned for more fun in the future!
Want to try the challenge for yourself? Use this text editor to find out if the words you choose are allowed or not! If you are a grad student doing chemistry-related research, we’d love to post your Ten Hundred Word Challenge on Chembites! Shoot us an email to get started – [email protected].
Many thanks to xkcd for inspiring people to take on this challenge, and to Science Buffs for keeping the challenge alive!