Title: Electrocatalytic Activity of Individual Pt Nanoparticles Studied by Nanoscale Scanning Electrochemical Microscopy
Authors: Jiyeon Kim, Christophe Renault, Nikoloz Nioradze, Netzahualcóyotl Arroyo-Currás, Kevin C. Leonard, and Allen J. Bard*
Publication Info: J. Am. Chem. Soc., 2016, 138 (27), pp 8560–8568 DOI: 10.1021/jacs.6b03980
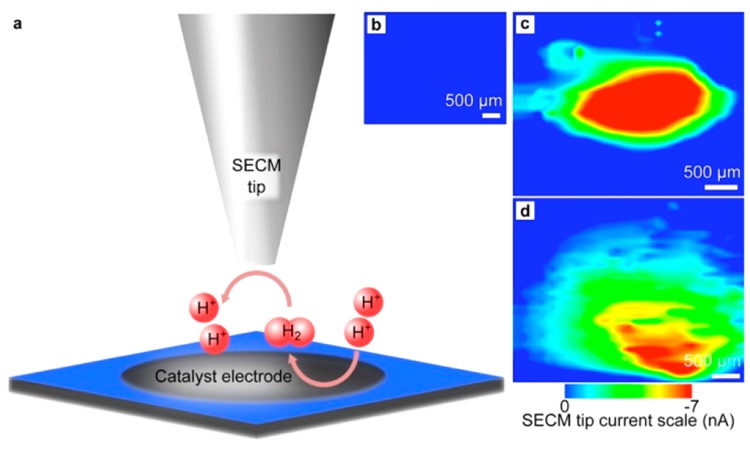
The mere mention of the word “nanoparticle” elicits awe and excitement in people in all walks of life. From their initial utilization hundreds of years ago where they enabled brilliant colors to emerge by infusion into glass and ceramics, to more modern applications such as their use for selective delivery of medication to individual cells, or to catalyze the combustion of fuel in our automobiles – nanoparticles are both revered and represent an active field of research. While catalytic properties induced by a large collection of nanoparticles have been well documented, quantitative analysis at the individual nanoparticle level has been lacking – and was the goal of the research addressed in this ChemBite. Specifically, a technique, and validation of said method, for performing electrocatalytic analysis of individual nanoparticles is presented in this work. This research succeeded through the complementary use of scanning electrochemical microscopy (SECM) employing nanoelectrodes to measure the electrocatalytic effects induced by individual nanoparticles, and atomic force microscopy (AFM) to characterize individual nanoparticle size and structure; thus quantifying structure-function correlations for individual nanoparticles and demonstrating a methodology that is conducive for extension to other nanoparticle systems as well.
The ability to perform quantitative structure-function analysis at the individual nanoparticle level is essential. For example, it is well documented that platinum nanoparticles can attain a greater catalytic effect for the electrochemical reduction of H2 than for the reaction performed instead with a bulk platinum electrode – evidenced by an increase in the rate constant for the reaction. One aspect that is typically ignored with nanoparticle systems is that it is likely that the impact from each individual nanoparticle is quite different than the bulk average due to slight differences in structure and composition of each individual particle (discussed more below). With the macroscopic analysis of nanoparticle systems that is typically reported, all that can be determined is an average collective effect from all of the nanoparticles, when it is actually quite possible that a small fraction of the nanoparticles are responsible for a majority of a beneficial result. By analogy, imagine a hypothetical situation in which you are tasked with analyzing widget production of Acme Widget Factory; consisting of 10 employees that collectively produce 100 widgets per day. Restricted to only this data, you could only conjecture about the collective average: 100 widgets are produced each day by the 10 employees. But assume you are also provided production data at the individual employee level; data that reveals there exists a single employee who produces 91 widgets per day, while the other 9 employees incompetently fiddle around and each only produce 1 widget per day. Such enhanced resolution is quite enlightening.
One aspect that makes nanoparticles so wondrous and remarkable is this fact that a mole of atoms arranged as individual nanoparticles exhibits remarkably different physical and chemical behaviors than the same mole of atoms arranged as a single entity. Likewise, a nanoparticle with pyramidal geometry may exhibit different properties than a spherical one; and other factors such as crystallinity, crystal structure and domain size, and even the density and type of defects (e.g. vacancy, interstitial, grain boundary defects) characterizing a specific nanoparticle will significantly affect its physical and chemical properties.
Although a comprehensive foundation in quantum mechanics is requisite to vitally engage in the field of nanoscience, without such one can still recognize the fascinating and unique characteristics exhibited by nanoparticles relative to their “bulk” counterparts. While overly simplified, many interesting attributes of nanoparticles can be rationalized by simple consideration of size and surface properties.
- Size effects: One defining trait of all nanoparticles is measuring 1000 nm or less in at least one dimension. Unique properties emerge from an object when its dimensions are scaled down similar to quantum mechanically defined characteristic lengths (e.g. exciton Bohr radius, electron mean free path, coherence lengths, etc.); properties which vary depending on an object’s atomic composition and structure. Spherical gold nanoparticles are exemplary of this; a solution of 25 nm gold nanoparticles appears ruby red, while a solution of 50 nm gold nanoparticles appears emerald green. Gold is not necessarily gold!
- Surface effects: The atoms at the surface of any object exist in a different environment than the interior atoms of that object. One significant and obvious differentiating characteristic of surface atoms is apparent in the context of bonding; where surface atoms are typically under-coordinated and the degree of unsaturation and arrangement significantly affects the surface energy and chemical reactivity. Recognizing significant differences exist between surface and bulk atoms, Figure 1 illustrates a critical feature of nanoparticles- a decrease in particle diameter results in an exponential increase in the fraction of surface atoms for a particle. For a spherical particle with a diameter less than 5 nm, a majority of the atoms composing the entire particle are surface atoms and thus a mass of atoms arranged as nanoparticles can exhibit millions of times greater surface area than that of the same atoms arranged as a single entity.