Title: Quantification, confirmation and screening capability of UHPLC coupled to triple quadrupole and hybrid quadrupole time-of-flight mass spectrometry in pesticide residue analysis
Authors: Susana Grimalt, Juan V. Sancho, Óscar J. Pozo, Félix Hernández
Published: 18 March 2010
Journal: Journal of Mass Spectrometry
http://onlinelibrary.wiley.com/doi/10.1002/jms.1728/abstract
In the previous article, we learnt about how food is prepared and treated before instrumental analysis (Check out for this article: How much pesticides are in your food? – Sample preparation in a nutshell). This article will be focusing on a very commonly used instrumentation, Liquid Chromatography-mass spectrometry (LC-MS).
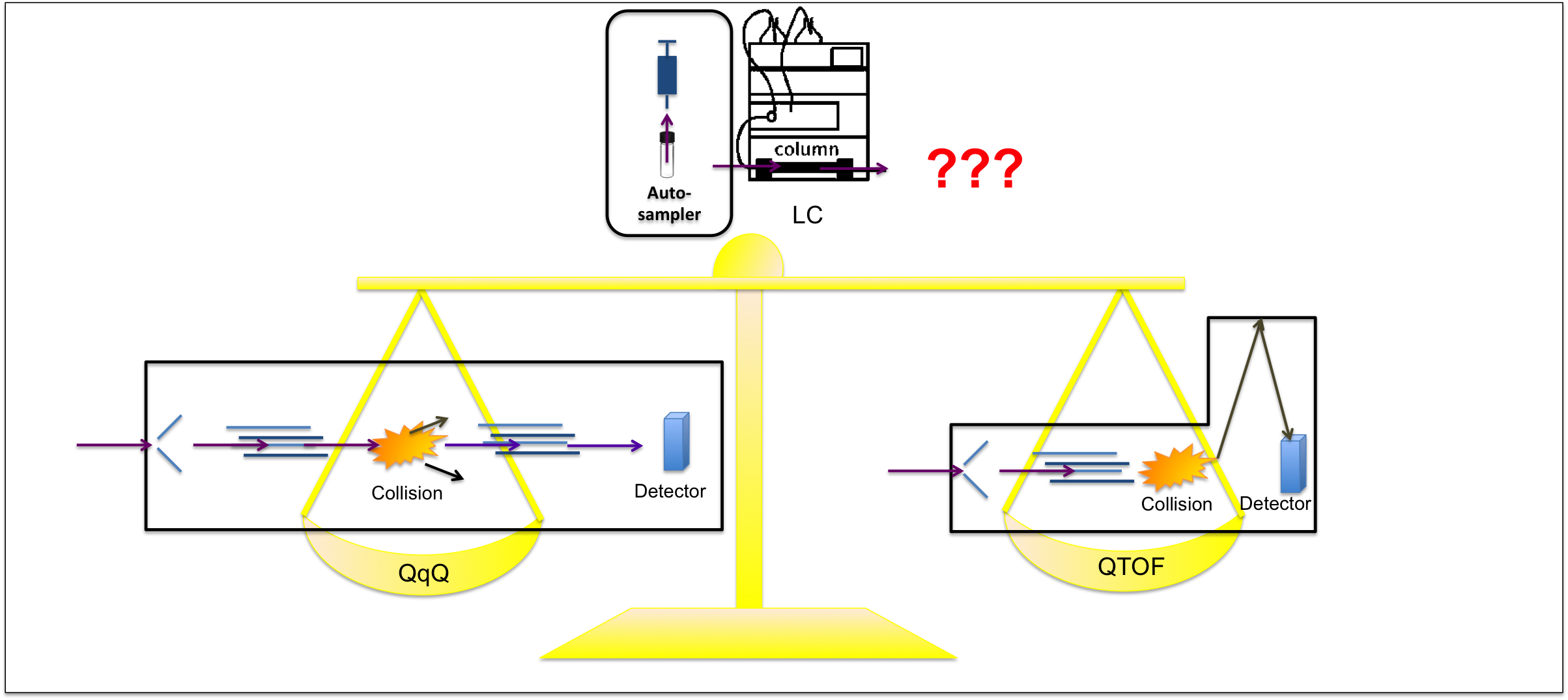
The name LC-MS already suggests that there are two components, LC and MS. Let’s look into the first puzzle, liquid chromatography, or LC. It is used for the separation of different compounds in a mixture. These compounds pass through a column with the aid of solvents. They are then sorted according to their order of elution. It could be thought of as a school to sort its hundreds of students according to their ranks in a swimming race. Some students preferred to play in the water while some students swim with their fastest speed hoping to get out of the water as quickly as possible. Just as each unique student has their unique speed, different compounds have their individual elution order according to their preference to solvents and column materials. Some compounds enjoy coupling with column materials longer while some compounds prefer to swim out with the solvent used.
So, LC allows us to know there are many compounds in a mixture, but how do we identify each of them? In MS, the compound is first ionised and the abundance of its fragmented ions are listed according to their mass-to-charge ratios. Each compound has their unique pattern of fragmented ions, just like fingerprints (Figure 1).

Figure 1 Examples of a MS chromatogram and the break down of each peak.
These are the basic principles of LC-MS, yet the field is full of variety. Resulting from the evolution of analyzer designs, triple quadrulpole (QqQ), time-of-flight (TOF) and quadruple TOF (QTOF) are developed and widely used in analysis of organic contaminants residues.
In a nutshell, QqQ is also considered as tandem MS (or MS/MS). As the name QqQ suggests, it has three quadrupoles (Q), where the first and the third ones acts as a gate to allow desired fragmented ions to pass through, while the second one behaves as a collision compartment. After the first quadrupole, the original compound is ionised, called the parent ion. It then enters the second quadrupole (q), which shatters the parent ions into smaller ions (daughter ions) and the third quadrupole guides these final daughter fragments to the detector. This can be analogue to a family tree. Just as we can trace from the daughters and sons to their ancestors, we can identify the compound by its specific daughter ions resulted from their particular parent ions. For TOF, it can be thought as replacing quadrupole (Q) by a time-of-flight analyzer, where the ions are sorted by their travelling time in the instrument. For QTOF, it can be analogy to a QqQ with the third quadrupole (Q) replaced by a TOF measurement.
This paper compares three kinds of LC-MS/MS: QqQ, TOF and QTOF with respect to their quantification, confirmative and screening capabilities.
In the realm of quantification, QqQ has a wider range for setting up linear calibration curves and a higher sensitivity compared to TOF and QTOF. Both QTOF and TOF presented similar sensitivity. Also, QqQ also possesses the lowest detectable levels for all analytes of interest in the study. Another weakness of TOF or QTOF is that their signals are more easily interfered by samples (Figure 2).

Figure 2 Chromatograms of grape sample with hexythiazox (a pesticide analyte) obtained by QqQ (left), TOF (center), QTOF (right). It can be seen that there are sample interference with TOF (high background signal). It is also noted that only two pair of ions are visible for QTOF while there are five for QqQ, demonstrating the better consistency of QqQ.
As for confirmation capacity, it can be measured by identification points (IPs), which is a system developed by European Commission in 2002. The IPs earned for each compound is related to the different MS techniques as well as its number of fragments. QTOF has the highest number of IPs for most compounds (Table 1), since fragments are formed efficiently during collision and their masses are accurately measured. Although QqQ has fewer identification points than QTOF, it demonstrates better consistency for all analytes at all levels tested, revealing its potential in confirmation capacity (Figure 2).

Table 1 Confirmation data obtained for real samples analysis. It can be seen that QTOF possesses the highest number of identification points, indicating its highest confirmation capacity.
With regard to screening, TOF outweighs the rest for its sensitivity in acquiring full spectrum scans with accurate mass measurements.
In short, this article presents a comprehensive study of the pros and cons for QqQ, TOF and QTOF instruments. It would be easier to understand why QqQ and QTOF are often used for target compounds analysis, while TOF is famous for unknown scanning in the industrial world.

Pingback:How a method is recognised as a method? – an example by a honey story – Eli's Science Blog
Pingback:Continuing the pesticide analysis journey: peeking into different LC-MS instrumentation – Eli's Science Blog