Title: One-Shot, reagent-free determination of the alcoholic content of distilled beverages by thermal infrared enthalpimetry
Authors: Juliano Barin et. al.
Publication Info: https://doi.org/10.1016/j.talanta.2017.05.011
During the manufacture of chemical products, the ability to rapidly and accurately monitoring the concentrations of various components is critical. For the alcoholic beverage industry, timely and accurate measurement of alcohol content is essential.
Traditionally this requirement has been satisfied by time-intensive techniques such as densimetry or gas chromatography which is relatively expensive and requires specialized training. In this report a new in situ technique, requiring only a basic infrared camera, is demonstrated to enable accurate and rapid (~10 seconds) quantitative analysis of alcohol content and requiring only small sample aliquots (~3 mL).
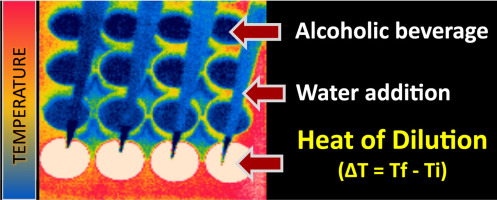
This method is facilitated by the phenomenon that dilution of alcoholic solutions is an exothermic process (see footnote below for further discussion of this), which results in

a change in system temperature. In this work the researches demonstrated that the magnitude of the temperature linearly correlated with a sample’s alcohol concentration. Non-contact temperature measurement, employing an infrared (IR) camera or thermometer, provides rapid results and further, averts potential contamination issues possible with contact-based methods.
This report is compelling beyond its specific goal of measuring alcohol content. The clever application of such a basic chemical concept, the heat of mixing/dilution, to solve a problem is inspiring. What other relevant problems can be solved by such fundamental chemical principles?
Footnote:
In general, the mixing of two substances or solutions results in some quantity of heat either being released to (exothermic), or absorbed from (endothermic), the surroundings. And this exchange of thermal energy reflects a change in enthalpy of the entire system. This is a familiar experience to most. For example, “instant” first-aid cold packs feel cool to the touch after the user breaks a barrier between two compartments and allows the separated contents to mix; typically enabling solid ammonium nitrate to mix/dissolve into water which is an endothermic process. This is a general behavior, but in most “household” cases the change in enthalpy by mixing things is too small to readily detect by touch.
Long ago it was recognized that when water and ethanol (alcohol) were mixed, the resulting mixture both felt warmer to the touch and contracted in volume by ~10%. This phenomenon has been continuously studied for over 100 years. New reports are regularly produced employing high levels of quantum mechanical theory and neutron diffraction experiments in order to refine the fundamental understanding of the water-alcohol structure and interactions responsible for these behaviors.
For those with even cursory exposure to chemistry and recognition of the structure of the water and ethanol molecules, it is intriguing to ponder, “why is mixing of ethanol and water an exothermic process?” In general, liquids are characterized as lacking any real long-range structural order, but exhibiting short-range order in the form of small clusters or networks. Unlike ideal gases, the arrangement of molecules in the liquid phase is not completely random.
the water and ethanol molecules, it is intriguing to ponder, “why is mixing of ethanol and water an exothermic process?” In general, liquids are characterized as lacking any real long-range structural order, but exhibiting short-range order in the form of small clusters or networks. Unlike ideal gases, the arrangement of molecules in the liquid phase is not completely random.
In pure liquid water or ethanol, their short-range order has been characterised as chains and/or ring-like structures typically consisting of of 6-8 molecules. Although these clusters are constantly breaking apart and reforming, the 6-8 molecules composing each cluster structure tends to almost exclusively exhibit intermolecular interactions predominantly within the cluster they exist, and exhibit minimal interactions with other clusters in the system. In mixtures of ethanol and water, it has been determined that the system is still predominantly composed of clusters of pure water and ethanol, but in the mixture the clusters of methanol and water exhibit strong attractive interactions between each other. Such additional intermolecular interactions provide an explanation for the lower enthalpy and decrease in density for the mixture.