Title: Unravelling the electronic structure and dynamics of an isolated molecular rotary motor in the gas-phase
Authors: Reece Beekmeyer, Michael A. Parkes, Luke Ridgwell, Jamie W. Riley, Jiawen Chen, Ben L. Feringa, Andrew Kerridge and Helen H. Fielding
Year: 2017
Journal: Chemical Science
http://pubs.rsc.org/en/content/articlelanding/2017/sc/c7sc01997a#!divAbstract
If you were asked to build a paper windmill, you may be rolling up papers as blades and picking a straw as the axle, giving it a push to enable it to rotate. Now these windmill-like motors can go microscale! Scientists have been building motors at the molecular level! And this achievement has been rewarded with the Nobel Prize in Chemistry of this year!
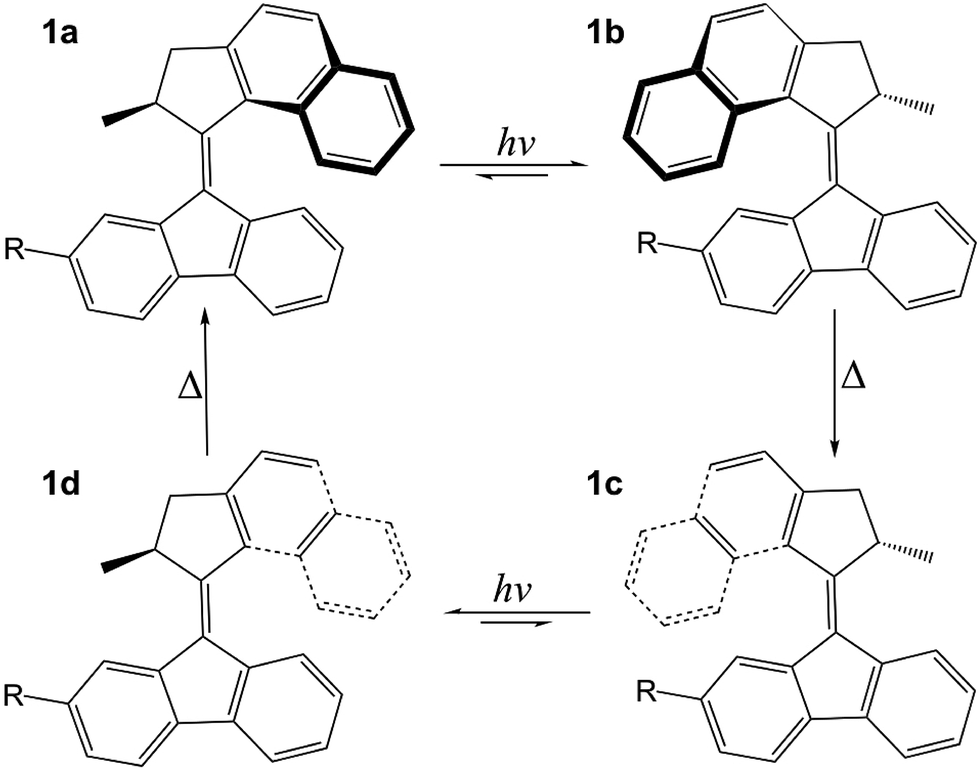
The principle to make a molecular motor is more or less similar to macroscale ones: find a blade, an axle and a driving force. In this article, the blade is made up of an overcrowded fluorene-based molecule (Figure 1), linked by an alkene axle and propelled by light. Sounds cool, but how does it work?
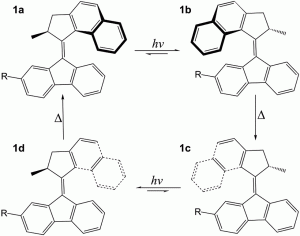
Figure 1 Structure of the molecular motor presented in the studies with R = COO−. “hν” represents the UV light that propels the motor, ∆ represents the energy released when the motor reorients itself to a more “comfortable” geometry.
At the stationary stage, the molecule seeks its most comfortable conformation (i.e. the geometry with the lowest energy). The methyl group places itself along the axle, as this is least crowded with the rest of the molecule. Then the molecule is “fired” by ultra-violet (UV) light, which rotates the molecule around the axle and forces the methyl group to an equatorial orientation. This causes much strain to the molecule, so the molecule situates itself to its favorable axial geometry. Then a second UV radiation is fired to keep the molecule in motion and complete its rotation. This is how a light-driven molecular motor works!
In order to create a faster and more efficient motor, it has to overcome two major obstacles. First, it is the conversion efficiency of the UV light to set the motor in motion (photochemical conversion efficiency). Second, it is the energy required for the molecule to orientate itself from the equatorial position to the axial position after the first UV light.
Scientists have been trying to measure the photochemical conversion efficiency of the molecular motor in solution phase with various spectroscopy techniques. Studies have shown that by attaching substituents with different properties, such as electron-withdrawing or electron-donating groups, to the central alkene could alter the efficiency without affecting the rotation frequency. Yet, the authors here want to study more about the intrinsic properties of the molecular motor, regardless of solvent interactions. So, they presented this study of the motor in gas phase.
They first reconstructed the molecule by replacing the methyl group (as in Figure 1) by the –COOH group and measured the electronic structure of the molecular motor with a spectroscopy called anion photoelectron spectroscopy. Basically, this involves kicking a hydrogen atom out from the –COOH substituent in the molecule (deprotonation) and taking many laser-images of it. On the other hand, they also performed computational calculations to simulate the electronic structure of the motor. Interestingly, they found that the molecule behaves similarly upon UV light radiation in both solution and gas state with anion photoelectron spectroscopy. On the other hand, since gas-phase calculations on molecules are usually less demanding for runtime and computational resources, this suggests calculations on photochemistry on molecular motor in gas phase could provide important benchmarks for understanding its motion at its “firing” stage.
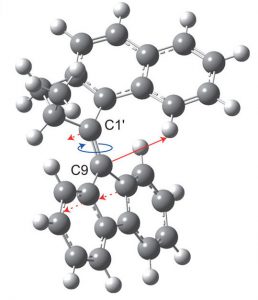
Figure 2 Illustration of the rotating motion (picture adapted from their earlier work).

Pingback:Build a motor, with molecules! – Eli's Science Blog