Title: Bioinspired Protein Channel-Based Scanning Ion Conductance
Microscopy (Bio-SICM) for Simultaneous Conductance and Specific
Molecular Imaging
Authors: Florika C. Macazo and Ryan J. White
Year: 2016
Journal: Journal of American Chemical Society
DOI: 10.1021/jacs.5b13252
Take a moment and recognize a few functions your liver performs – it cleans your blood, regulates glycogen storage, and produces digestive chemicals. Such tasks are not realized by each of the ~100 billion liver cells individually performing one-hundred-billionth of all liver task, the realization is synergistic and emerges from the collective collaboration of an ensemble of cells. Likewise, the ~40 trillion cells that compose you, are not just 40 trillion tiny versions of you; your “you-ness” is also synergistic and is an emergent property. For complexity to emerge in multi-cellular organisms, extensive intercelluar communication must occur.
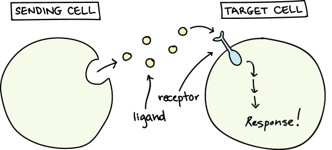
Intercellular signaling occurs via mechanical, electrical, light driven, and chemical means, but the research highlighted here, from Ryan White at the University of Cincinnati, focuses specifically on chemical signaling processes as depicted in Figure 1. Hundreds of different chemical signaling molecules have been identified in humans with three major classifications: neurotransmitters (e.g. dopamine, serotonin), hormones (e.g. insulin, estrogen, etc.), and cytokines (interferons, interleukins, etc.) – and hundreds more molecular species typically exist in extracellular fluid. For effective intercellular communication to occur, receptors must be able to selectively identify and bind only a specific and single signaling molecule within a complex matrix. This selective recognition exhibited by the membrane receptor proteins of cells is quite amazing. This is on par with two people sitting at opposite ends of a packed stadium, in the midst of 70,000 screaming fans, and able to verbally communicate with each other – and cells can do this!
Recognition that various faults in the intercellular signaling processes are often the culprits for a number of common diseases (such as diabetes, multiple sclerosis, muscular dystrophy, Parkinson’s, and even some cancers), study of intercelluar communication processes are readily warranted. Just one component of these studies involves monitoring intercellular signalling at the individual cell and single molecule level. Such an endeavor is challenging since human-designed bio-sensors are typically not selective or sensitive enough for many signaling molecules to achieve the desired temporal and concentration profiles for a single type of signaling molecule – and this work presents an ambitious and promising solution to this problem.
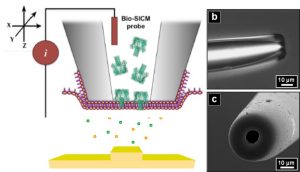
As illustrated in Figure 2, the White research group has approached this problem by recognizing and exploiting the 3 billion years of evolutionary trials nature has performed in attaining selectivity and sensitivity for such signaling molecules. In essence, they “cap” the orifice of small, glass capillaries with cell-like bilayer lipid membranes which provides an environment conducive for the incorporation – and necessary for functionality- of membrane receptor proteins. By choosing to specifically incorporate ligand-gated ion-channel membrane proteins (i.e. receptors that open an ion channel upon binding with a specific signaling molecule) into their sensors, the electrical conductance through the capillary is modulated (and readily detectable) by binding between the incorporated membrane receptor and a specific signaling molecule. As these capillaries are on the order of a micrometer in diameter, they function as probes to map out the temporal and spatial chemical signaling profiles of individual cells. While this work predominantly functions as a proof-of-concept for the technique, it demonstrates the potential to selectively obtain high-resolution, spatial and temporal profiles of effectively any intercellular signaling molecule of interest.