Title: Adsorption of iron oxide nanoclusters stabilized with sulfonated copolymers on silica in concentrated NaCl and CaCl2 brine
Authors: Hitesh G. Bagaria , Bethany M. Neilson , Andrew J. Worthen , Zheng Xue , Ki Youl Yoon, Victoria Cheng, Jae Ho Lee, Sindhuja Velagala, Chun Huh, Steven L. Bryant, Christopher W. Bielawski, Keith P. Johnston
By: Ehsan Moaseri
Accurate and non-invasive determination of oil saturation distribution and bypassed oils can significantly improve the enhanced oil recovery (EOR) processes. This improvement would lead to a major reduction in the extraction cost. As current techniques, including NMR lodging and partitioning tracers, are inefficient and not highly reliable, the need for a new effective imaging technique is appreciated more than ever in the oil industry.
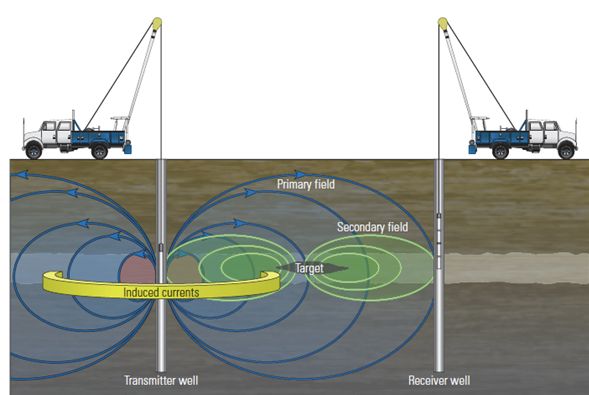
Bryant and Johnston groups have proposed a new technique using paramagnetic Iron Oxide nanoparticles (IONs) as contrasting agents. In this technique, IONs are injected into the reservoirs and thereafter adsorbed at the oil-water interface or dispersed in one of the fluid phases in reservoir. An external magnetic field is envisioned to be applied and cause the IONs to vibrate, displace the interphase, and emit a pressure (acoustic) wave. This acoustic wave can be detected by the sensors and transformed into an image.
In oil reservoirs, high salinities are often encountered. For example, NaCl concentrations are usually >5 wt. % (0.9 M) and CaCl2 concentrations may reach 1–2 wt.% (90– 180 mM). The high salinity and the strong attraction forces between the IONs reduce the stability of the nanoparticles and cause aggregation. ION aggregates show poor transportability in the porous media of the reservoirs and as a result, imaging cannot be done if the nanoparticles are aggregated. Therefore, introducing an effective method to stabilize the IONs in these harsh conditions is very challenging and crucial to apply this technique to real field trials.
In this paper, stability of the IONs in harsh reservoir conditions and their transport in porous rock is studied. The stability of the IONs was greatly enhanced by attaching polymer brushes on the surface of these nanoparticles; these brushes would prevent aggregation of the IONs; whenever two nanoparticle become close to each other, the brushes would push them back and prevent aggregation. Various polymers composed of acrylic acid and either 2-acrylamido-2-methylpropanesulfonate or styrenesulfonate were synthesized and adsorbed on IONs to provide colloidal stability.
It was found that the negatively charged 2-acrylamido-2-methylpropanesulfonate can adequately stabilize the nanoparticles and also provide the required transport in the porous media. The negative charge of the polymer brushes also restrict the adsorption of the IONs on the underground rocks. This lack of adsorption was attributed to the electrostatic repulsion between the polymeric brushes and the negatively charged silica surface of the rocks.
It was found that many other classes of polymers could not stabilize the IONs in the harsh conditions of the reservoirs, including the high salinity and elevated temperature. This can be explained by the interaction of the polymers with the solution; in high salinities, the polymer chains cannot remain extended and they would collapse. The collapsed brushes cannot provide the required repulsion to prevent the aggregations. The 2-acrylamido-2-methylpropanesulfonate, on the other hand, can remain extended even in the high salinities due to the very strong acidity of the sulfonate group.