Title: Surfactant Ligand Removal and Rational Fabrication of Inorganically Connected Quantum Dots
Authors: Haitao Zhang, Bo Hu, Liangfeng Sun, Robert Hovden, Frank W. Wise, David A. Muller, and Richard D. Robinson
Publication Journal and link: Nano Lett., 2011, 11 (12), pp 5356–5361 (10.1021/nl202892p)
Chemistry is the science of understanding existing materials and blending the knowledge gained to synthesize new materials with novel properties. There are four primary parameters that can be tuned to get different materials with different properties:
- Chemical composition – What is the material made of? Which atoms from the periodic table are present?
- Structure – How are the atoms arranged in the material?
- Bonding – How are the atoms connected to each other?
- Size – How big is the material?
By tuning these four parameters chemists have discovered a lot of different materials which previously were unknown or didn’t exist. One such revolutionary discovery was the synthesis of nanoparticles. Nanoparticles, as discussed in previous articles, have unique properties because of their small size, which have changed the face of science, technology, and medicine.
Due to tremendous potential of nanoparticles, scientists are exploring another dimension in nanoparticle research.
Currently, nanoparticles are used in 3 forms: (a) embedded in a matrix like seeds in a watermelon (b) dispersed in solution like pebbles in water and (c) assembled on a substrate like jam on bread. In all the three cases the nanoparticles are not connected to each other, as shown in Fig. 1. In case (a) the matrix is between nanoparticles and in (b) and (c) there are surfactant molecules between nanoparticles.

Surfactant molecules are long chain organic molecules which are added to nanoparticle surface to stabilize them. Essentially, nanoparticles have a huge surface area and small volume due to their small size. The atoms on the surface are different from the bulk material because on one side they do not have neighboring atoms as shown in the Fig. 2. These missing neighbors lead to unsatisfied bonds on the surface which make nanoparticles unstable. To reach a stable state the nanoparticles want to come together and aggregate, forming a bulk material.
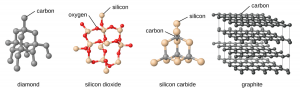
Therefore, surfactant molecules are used to satisfy the unsatisfied bonds on the surface of a nanoparticle and prevent them from aggregating. The matrix also does a similar job of stabilizing nanoparticle surface by surrounding it. Though the surfactant molecules/matrix stabilizes nanoparticles, they prevent nanoparticles from interacting with each other. However, if you think of a bulk material as shown in Fig. 3, the atoms are connected to each other by chemical bonds. These bonds facilitate the motion and interaction of electrons of different atoms. Consequently, this structural arrangement of atoms and their chemical connectivity dictates the property of the material.
So, if we were to think of nanoparticle as an atom building a nanostructure, the presence of chemical bonding between them could lead to a new class of materials which combine the unique properties of nanoparticles with the structure and connectivity of bulk materials.
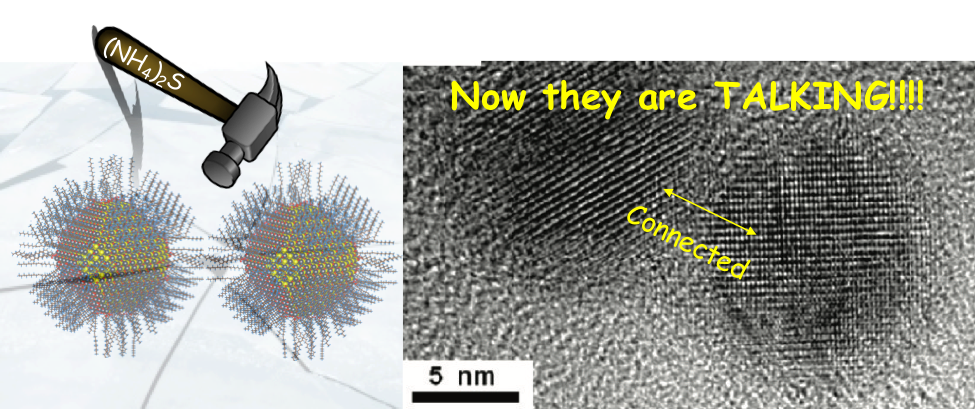
In the article we are discussing today, the researchers have found a way to make nanoparticles talk to each other by removing surfactants on the nanoparticle’s surface. Earlier attempts to remove surfactants by heating led to aggregation of nanoparticles. Researchers also tried to exchange the surfactants on the surface with smaller ones but this still prevented nanoparticles from chemically bonding. In the current approach, ammonium sulphide, (NH4)2S, is used to remove the surfactants. (NH4)2S is a metal free salt that reacts vigorously with the inorganic surfactants. This method was tried on lead sulphide (PbS) and cadmium selenide (CdSe) nanoparticles. Both PbS and CdSe are semiconductor nanoparticles also known as quantum dots (QDs). Semiconductors are materials which have electrical conductivity between a conductor and an insulator. This intermediate conductivity arises due to presence of a band gap, the energy gap that electrons need to overcome to flow freely and conduct electricity. QDs are semiconductors in nanometer dimension and also have a band gap. The size of the QD decides its band gap. QDs are widely used in electronic devices like quantum dot TVs which has high color purity due to CdSe color panel.
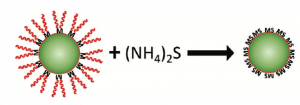
The films of these QDs were allowed to react with the (NH4)2S solution, which removed the surfactants and converted the metal rich surface layer into metal sulphide in a few seconds (see Fig. 4). These metal sulphides then formed chemical bonds between the QDs.
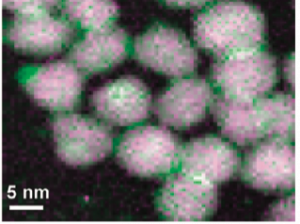
The researchers confirmed the formation of connected QDs using Transmission Electron Microscopy (TEM). Given the small size of nanoparticles it is not possible to see them by our naked eyes.
Therefore, we use a special microscope which uses the interaction of electrons with the material to generate an image. Fig. 5 shows TEM images of PbS QDs before and after (NH4)2S treatment. Similar results were seen for CdSe QDs as shown in Fig. 6.

After establishing the formation of structure, it is important to determine if this structural connectivity compromised the unique properties of QDs. To understand this, the band gap was probed using absorption spectroscopy. Like we had discussed before, quantum dots are semiconductor nanoparticles which have a band gap. This band gap is the energy gap between the highest occupied energy level by electron and lowest unoccupied energy level. So, if sufficient energy is given to the electron in the occupied level, it can jump through the gap to the unoccupied level as shown in Fig. 7.
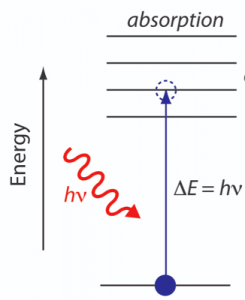
So, if we solve this problem backwards we can find the band gap. Light of different energy is incident on the QD film and the absorption of light is recorded as a function of its energy. This experiment is called absorption spectroscopy and the spectrum obtained is called an absorption spectrum. When the energy of light is greater than or equal to the band gap energy, electrons will absorb it and jump to higher energy level creating a peak in the spectrum. The position of the peak gives the band gap.
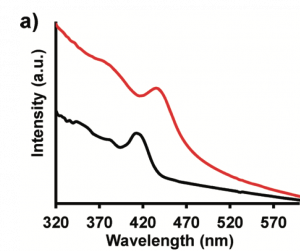
Even with the interconnected structure, the band gap should remain nearly the same after the (NH4)2S treatment. Fig. 8 shows the absorption spectrum of CdS QDs which confirms no significant change in band gap. Thus, the absorption experiment showed that the researchers were successful in combining the strength of nanomaterials with the structure of the bulk materials without compromising the unique properties and paving ways for new novel physics.
In summary, a new class of materials called interconnected nanostructures is gaining a lot of attention in the scientific community. Preserving the identity and unique properties of nanoparticles, this material offers advantages by having connected structure give rise to new phenomena. One such method of connecting nanoparticles is treatment of nanoparticles by (NH4)2S which removes the surfactants on nanoparticle surface and allows them to bond. Research is underway to characterize the properties of these new materials.