Feature image by CSIRO shared under creative commons license: https://creativecommons.org/licenses/by/3.0/deed.en
The current Australian bushfire season has caused unprecedented damage and devastation, worse than any fire season the country has seen before. An estimated 5.4 million hectares have burnt since September last year, more than 14 times the area that burned in California in 2018. At least 34 people have died, hundreds of million animals have lost their lives or their habitat and around 2,500 homes have been destroyed. And the season isn’t even over yet.
The causes of these fires are varied and complex. But what role has chemistry played throughout this disaster?
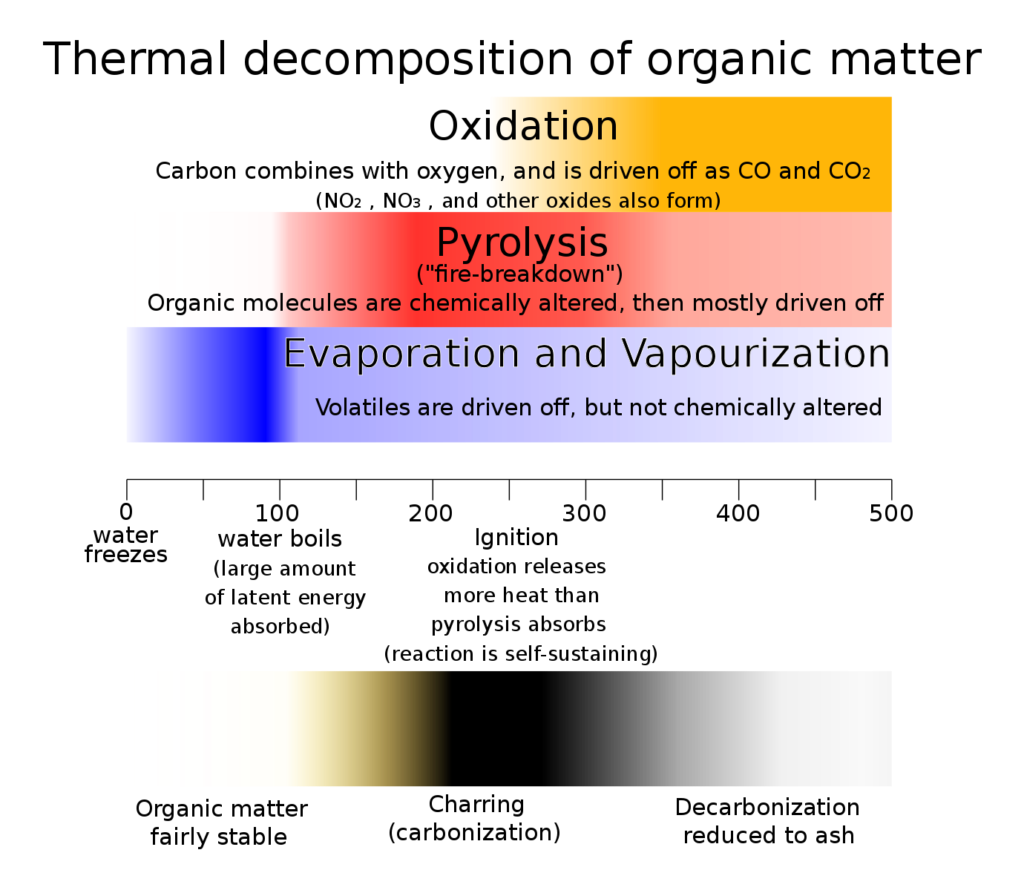
The most obvious chemical reaction associated with fire is combustion. The combustion reaction is a redox reaction in which a fuel burns, generally in oxygen, and produces heat as well as oxide products. In the case of wood, there are two key stages that occur before combustion: thermal degradation and ignition. Thermal degradation occurs as wood is heated to high temperatures, leading to dehydration and bond breaking within molecules such as cellulose and lignin, releasing further heat energy. This thermal degradation leads to the production of volatile gases, tar and carbonaceous char, a process known as pyrolysis. As pyrolysis continues and the heat increases in the presence of oxygen, the wood can ignite and combustion occurs.1
In Australia, Eucalyptus trees make up a large portion of the country’s tree flora. Unfortunately, their oil, known for its pleasant fragrance, is extremely flammable.2 When their leaves undergo pyrolysis, the amount of flammable gas that is released is immense and can cause rapid and explosive ignition.3,4 They also drop a lot of leaves and bark, meaning there is a lot of fuel on forest floors. Eucalyptus trees give true meaning to the phrase “spread like wildfire”.

The smoke produced by wood combustion contains particulates and a range of chemicals. These include both inorganic and organic compounds such as carbon monoxide, nitrogen- and sulfur-containing compounds, aldehydes, and polyaromatics.5 Inhalation of this smoke can cause severe respiratory irritation, impaired macrophage function, increased production of pro-inflammatory mediators as well as a range of other serious health problems.6 Some studies have shown that inhaling bushfire smoke can be as damaging as smoking cigarettes, but further research into the long-term health outcomes is required.7 This season of Australian fires has caused huge plumes of dangerous smoke in many parts of the country, this smoke has travelled all the way around the world and has even been visible from space.

https://creativecommons.org/licenses/by-sa/2.0/
Although the chemistry behind these fires is quite frightening, chemistry can also be used to help fight them. Fire retardants are substances containing a range of different chemicals that firefighters can use to help prevent the spread of wildfires, reduce fire intensity and to protect structures. They are generally released aerially from firefighting planes and are coloured red by iron oxide. There are many different types of fire retardants which may work to reduce the flammability or amount of fuel available, delay fuel combustion, create a glassy barrier on potential fuel sources or to achieve intumescence.8,9

The fire retardants most commonly used to fight Australian bushfires generally work to reduce the flammability and amount of fuel available to the fire. These retardants are manufactured as dry powders and then diluted with water to form a slurry. They are composed of: foaming agents, ammonium phosphate and ammonium sulfate salts, thickening agents and corrosion inhibitors.10 The phosphate and sulfate salts decompose to release phosphoric and sulfuric acid which can catalyse the decomposition of cellulosic materials, thus reducing the temperature required for thermal degradation. This increased level of thermal degradation at a lower temperature leads to an increase in CO2 and char production. The increased concentration of CO2 dilutes the amount of oxygen in the immediate environment, while the increased char leads to a reduction in the amount of fuel available.11,12 In these conditions, ignition and combustion become far less likely.
There is always science at the heart of natural disasters. It is a deep understanding of this underlying science that is and will continue to be used and innovated to help fight and prevent such disasters.
References
1. Lowden, L.A., Hull, T.R. (2013) Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci Rev 2, 4. https://doi.org/10.1186/2193-0414-2-4
2. Grootemaat S., Wright Ian J., van Bodegom P.M., Cornelissen J.H.C., Shaw V. (2017) Bark traits, decomposition and flammability of Australian forest trees. Aust J Bot 65. https://doi.org/10.1071/BT16258
3. Tumino B.J., Duff T.J., Goodger J.Q.D., Cawson J.G. (2019) Plant traits linked to field-scale flammability metrics in prescribed burns in Eucalyptus forest. PLoS ONE 14, 8. https://doi-org.ezproxy1.library.usyd.edu.au/10.1371/journal.pone.0221403
4. Gill, M., Zylstra, P. (2005) Flammability of Australian forests. Aust For. 68. doi:10.1080/00049158.2005.10674951
5. De Vos, A.J.B.M., Reisen, F., Cook, A. et al. (2009) Respiratory Irritants in Australian Bushfire Smoke: Air Toxics Sampling in a Smoke Chamber and During Prescribed Burns. Arch Environ Contam Toxicol 56, 380. https://doi.org/10.1007/s00244-008-9209-3 (respiratory effects)
6. Hamon, R., Tran, H.B., Roscioli, E. et al. (2018) Bushfire smoke is pro-inflammatory and suppresses macrophage phagocytic function. Sci Rep 8. https://doi.org/10.1038/s41598-018-31459-6
7. Krimmer D., Ichimaru Y., Burgess J., Black J., Oliver B. (2013) Exposure to Biomass Smoke Extract Enhances Fibronectin Release from Fibroblasts. PLoS ONE 8, 12. https://doi.org/10.1371/journal.pone.0083938
8. Camino, G., Luda, M.P. Mechanistic study of intumescence (1998), p. 48 f, in Le Bras, M.; Camino, G.; Bourbigot, S.; Delobel, R. Eds., Fire retardancy of polymers; The use of intumescence, The Royal Society of Chemistry, Cambridge, UK. Retrieved from http://books.google.com.
9. Green, J. (1996). Mechanisms for Flame Retardancy and Smoke suppression -A Review. J. Fire Sci. 14, 6. https://doi.org/10.1177/073490419601400602
10. New South Wales Department of Health (2019) Fire Retardants and Private Water Sources. Retrieved from https://www.health.nsw.gov.au/environment/factsheets/Pages/fire-retardants.aspx
11. Mohamed A.L., Hassabo A.G. (2015) Flame Retardant of Cellulosic Materials and Their Composites. In: Visakh P., Arao Y. (eds) Flame Retardants. Engineering Materials. Springer. Retrieved from http://books.google.com.
12. Maurer, A., Staendeke, H., (1985) United States Patent No. 4,515,632. Retrieved from https://patents.google.com/patent/US4515632A/en

