Featured video is a clip from “kinetiX” by MIT Media Lab under CC BY-NC-ND 4.0
Title: Colossal Negative Linear Compressibility in Porous Organic Salts
Authors: Yu Zhao, Changzeng Fan, Cuiying Pei, Xu Geng, Guolong Xing, Teng Ben, Shilun Qiu
Journal: Journal of the American Chemical Society
Year: 2020
Some materials don’t react how you would expect when touched. Many people can recall when they first touched “oobleck,” a suspension of cornstarch in water, and instead of their hand dipping into the liquid, the material seemed to transform into a solid. Oobleck is a non-Newtonian fluid meaning that it does not obey Newton’s law of viscosity, which says that viscosity does not change when stress is applied to the material. This classification is not unique to oobleck, and it even applies to more commonplace materials like Ketchup and blood, though the effect is not as obvious.
If you look around for more unintuitive chemical properties, you will surely find them. Most materials shrink when pressure is applied, but chemists have designed materials that instead elongate along at least one axis (1 vs 2 in Figure 1, respectively). The latter are known as NLC materials, where NLC stands for negative linear compression. Linear compressibility is simply a measure of the rate of contraction in one dimension as pressure is applied. Materials that behave intuitively and shrink when pressure is applied have what is known as positive linear compressibility, and those that elongate along at least one axis have negative linear compressibility.
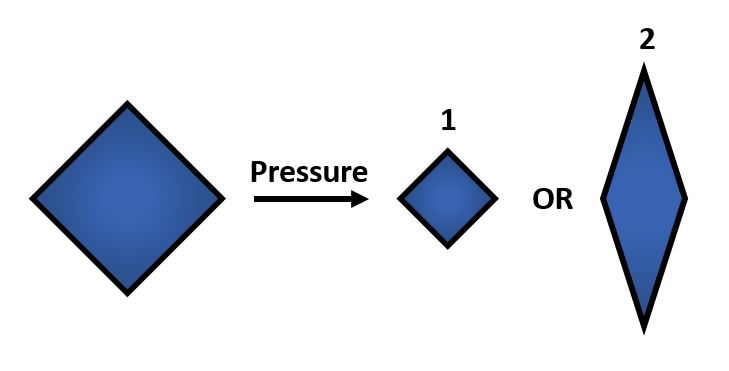
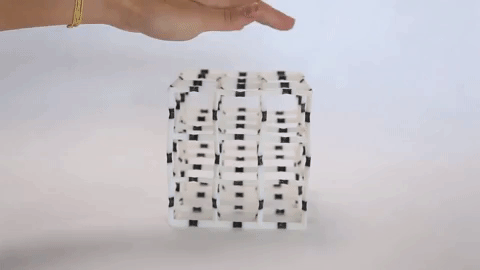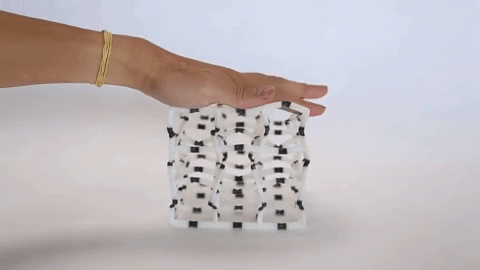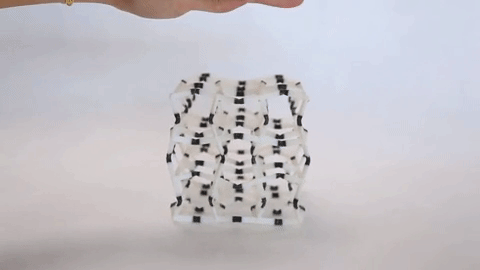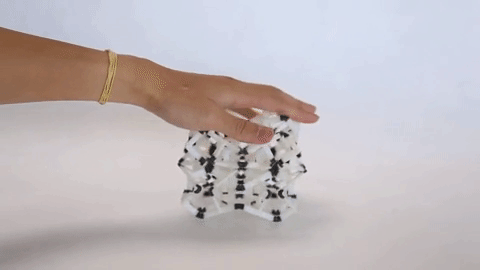
These materials are not just fascinating novelties but have practical uses as well. The incredible capacity of NLC materials to expand in a directional way under pressure could make them useful as artificial muscles, and the fact that they have a measurable response to pressure means they have potential for use as pressure sensors. Additionally, the same principles that are used to design architectures on the molecular scale are used to design larger scale materials that can flex and change shape, as is seen in the video at the top of the page.
Negative linear compressibility has been described before as being large or giant, but never has it been described as colossal, as it has for the newest member of this class of materials. This material, known as “CPOS-1” which stands for crystalline porous organic salt, achieves such a negative linear compressibility by employing a 3D rather than a 2D design. NLC materials that work in two dimensions often rely on a “wine rack” motif. If you were to apply force to one side of a wine rack as can be seen in Figure 2, it would compress in the perpendicular direction.

Instead of the 2D rhombuses seen in a wine rack, CPOS-1 employs 3D hexagons in a diamond-like pattern, as can be seen in Figure 3. For those familiar with introductory organic chemistry, these hexagons resemble the “chair” conformation of cyclohexane. CPOS-1 is made up of two building block molecules: tetrakis(4-sulfophenyl)methane and trans-1,4-diaminocyclohexane. At each corner of the diamond structure is a tetrakis(4-sulfophenyl)methane molecule, and the corners are connected by one trans-1,4-diaminocyclohexane molecule in between them. These two components are held together by strong N–H+···–O–S hydrogen bonds, which are formed by the complementary ionic charges on the sulfonate anions and ammonium cations on each molecule. The strength of CPOS-1 comes from the functioning of these connections as helical molecular springs which remain intact despite variation in the relative orientation of the molecules as the crystal is compressed.
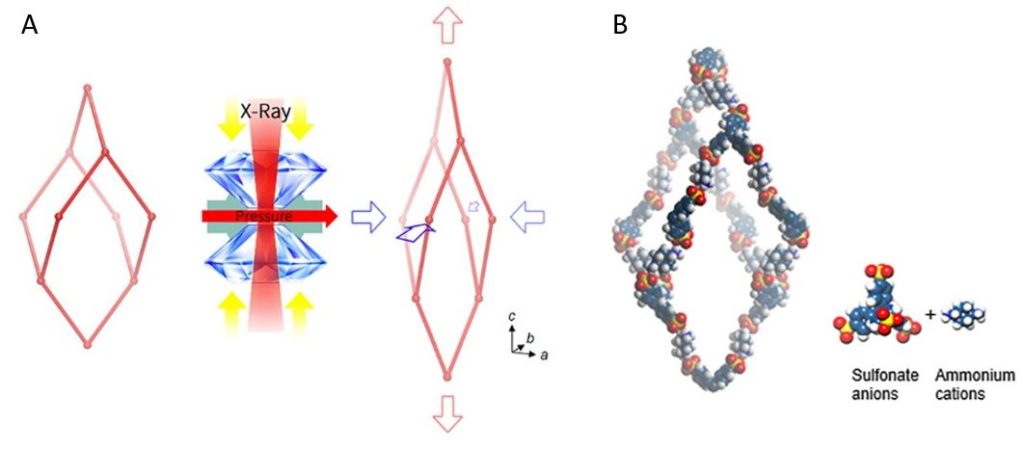
The design of CPOS-1 also addresses practical concerns that come with previously made NLC materials. Namely, these materials all have some combination of toxicity (cyanide-containing), reliance on precious metals (gold or silver), or are difficult to synthesize. Not only does CPOS-1 have the highest magnitude of negative linear compressibility ever reported in the literature, but it is non-toxic and easy to synthesize. Its synthesis includes two simple steps: mixing solutions of the two starting materials, and letting the solution sit for a few days while crystals of CPOS-1 grow.
The incorporation of innovative principles like 3D design into NLC materials may allow what has been a historically empty category of materials to finally flourish. Borrowing the concept of molecular springs from the field of molecular machines makes one wonder what other design principles may be incorporated into future pressure-responsive materials. What adjective will be used to describe NLC materials next after large, giant, and colossal?