Authors: Adewale George Adeniyi, Joshua O. Ighalo, Damilola Victoria Onifade
Journal: Chemistry Africa
Year: 2020
Featured image by esudroff from Pixabay
—
Picture a wood fire, logs set alight. Flames appear, the wood shrinks, eventually only some ashes remain. But where did the rest of the mass of the wood disappear to? Well, the carbon in the wood reacted with oxygen in the air, forming gaseous carbon dioxide—the combustion reaction. If, however, you were to heat the wood in an enclosed, oxygen-free environment instead (a process called pyrolysis), you could prevent the combustion reaction from occurring. As a result, instead of gas and ashes, you would get a solid carbon-based material we usually call charcoal. You might be familiar with charcoal as a convenient fuel, whether for home cooking or other industrial uses.
But saying that charcoal makes a good fuel is like saying that an iPhone makes a good paperweight… it’s true, but the potential uses of charcoal are astronomically more impressive than fuel alone. Charcoal has the potential to revolutionize the way we grow food, the way we produce and purify chemicals, our ability to clean up the environment, and even the kinds of materials we make our electronics out of. When charcoal is used for these purposes, it commonly goes by another name: biochar. Western Africa is one of many regions of the world where scientists have taken a particular interest in biochar; today, we’ll take a look at the work of researchers from Ilorin, Nigeria, on the potential applications of biochar made from orange peel waste.
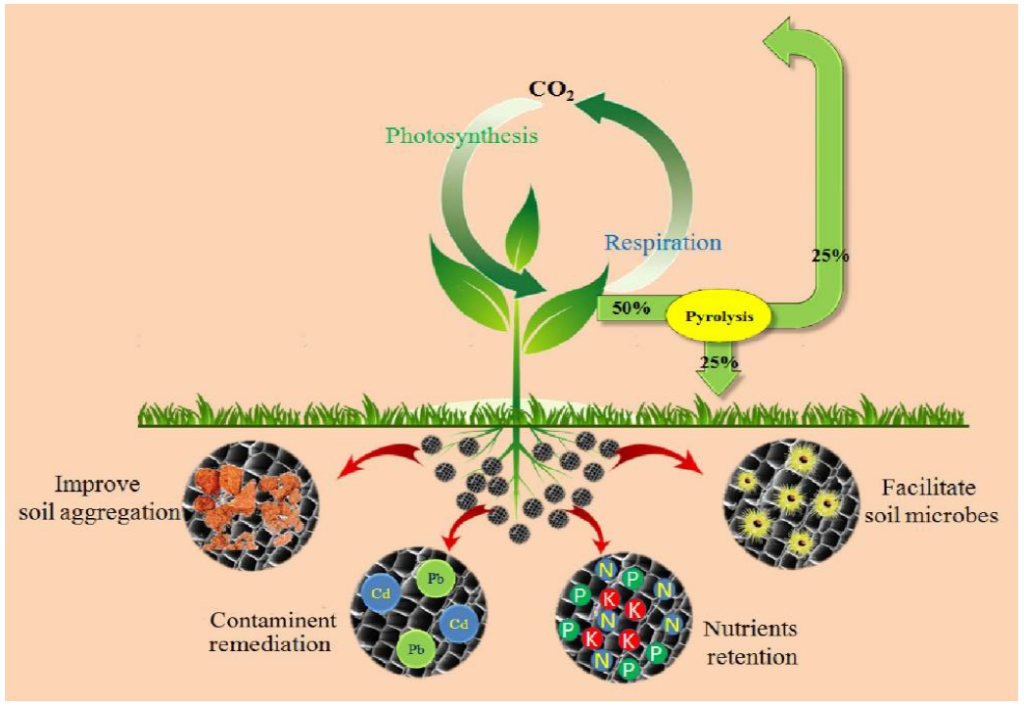
While the feedstock (i.e. the initial raw material) used to make fuel charcoal is traditionally wood, charcoal/biochar for agricultural or other purposes can be made from nearly any source of biomass. However, that doesn’t mean that all biochar turns out exactly the same. Different starting materials, depending on the specific chemical composition and structure, can have drastic effects on the resulting material properties. Part of what gives biochar its usefulness is its porous structure. When added to soil, the many pores hold water, and provide an environment for beneficial microorganisms to grow and accumulate nutrients, helping crops grow (Figure 1). Biochar’s porosity, plus the varying chemical functional groups on its surfaces, can also facilitate certain catalytic reactions or adsorption (i.e. deposition onto a surface—in this case, the biochar surface) of other chemical compounds. As a result, biochar may also be used in environmental remediation efforts to pull contaminants from local soil or water, thereby preventing people, plants, or animals from ingesting or absorbing them.
Due to the wide variety of possible biochar feedstocks, there is still a lot of work to be done to find out how both feedstock type and production process affect the end product and—therefore—the types of applications the resulting biochar is suitable for. In this study, Adeniyi et. al. investigated and compared the resulting characteristics of biochar made from orange fruit peels versus orange albedo (i.e. the white chewy bits everyone picks off before eating). As common food waste residues in many parts of Nigeria, both peel and albedo could serve as convenient local biochar feedstocks.
The researchers used a variety of methods to characterize different aspects of the biochar: they looked at the surface texture and elemental distribution using scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS); they identified the different chemical bonds present in the material (and therefore the different surface functional groups) with infrared spectroscopy (IR); and lastly, the pore volume and size distribution were determined by analytical methods involving the adsorption of an inert gas on the biochar surface.
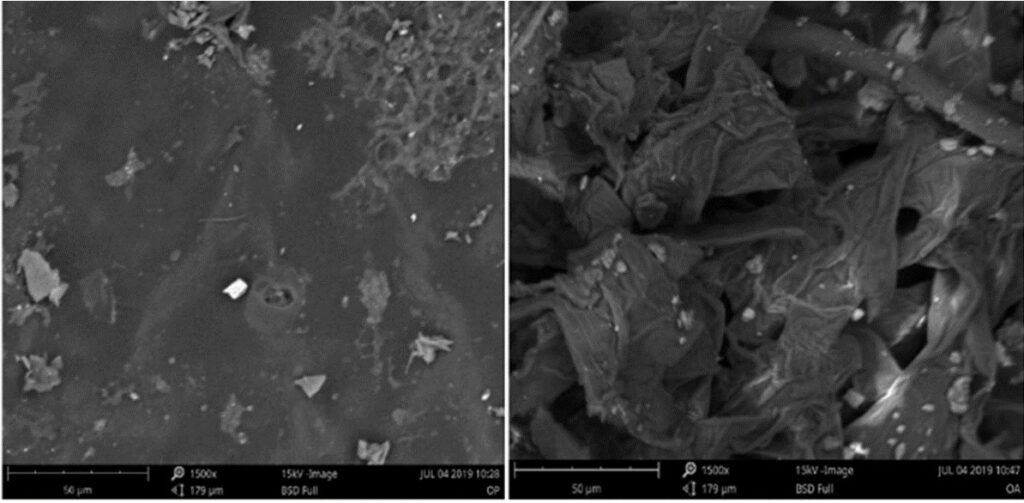
The SEM images revealed much smoother surfaces on the peel biochar compared to albedo which looked more “spongy” and porous (Figure 2). This would indicate that albedo biochar might be better suited for chemical adsorption and water retention applications. However, through physical measurements of pore volume the researchers found that despite the visibly higher surface area of the albedo, the peels biochar was actually slightly more porous overall! But that’s not the whole story; after all, chemical makeup matters as much as physical structure. In biochar, high carbon content and the presence of polar functional groups are also highly desirable for pollutant adsorption applications. Happily, EDS and IR results showed that peel as well as albedo biochar had these properties, and therefore both would be viable feedstock options.
Biochar, in its many variations, is a material with untold potential for creating more efficient and sustainable methods for growing food, decontaminating the environment, and producing clean energy. The work done by Adeniyi et. al. is part of a large and necessary push to understand the varying properties of biochar produced through different feedstocks and methods. Thanks to their work and others, step by step we are developing ways of living healthier and more sustainably on our planet.

