Manuscript: Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. Jill Hahn, Nancy R Cook, Erik K Alexander, Sonia Fridman, Joseph Walter, Vadim Bubes, Gregory Kotler, I-Min Lee, JoAnn E Manson, Karen H Costenbader. The BMJ. 2022.
Randomized controlled clinical trials are considered one of the best ways to generate data for conducting evidence-based medical treatment. Clinical trials are used to test the effects of drugs or other chemicals such as supplements. In this study, Hahn et al. examined the effects of supplementation of vitamin D and/or omega 3 fatty acids on the development of autoimmune disorders over time. Both chemicals have mechanistic implications for ameliorating immune disorders; vitamin D regulates genes involved in inflammation and immune response, while omega 3 fatty acids are thought to decrease systemic inflammation. However, because disease development is complex and almost always dependent on multiple factors, supplementation is not necessarily beneficial even if the chemicals are known to be important in the mechanisms of normal immune pathology. Therefore, clinical trials are vital for examining both the benefits and risks of supplementation. Let’s break down the description of this study, which is noted as “a nationwide, randomized, double blind, placebo controlled trial with a two-by-two factorial design,” to understand how these types of trials are conducted.
First, let’s look at what two-by-two factorial design is, as it will give clarity to the rest of the design. Two-by-two factorial design allows researchers to compare multiple independent variables. In this case, the variables are supplementation of vitamin D and/or supplementation of omega 3 fatty acids. Participants in the placebo group for vitamin D do not receive active vitamin D and instead receive a placebo pill. Similarly, if a participant is not taking omega 3 fatty acids they will take a visually identical but chemically null pill. Thus, each category has two options: active vitamin D or placebo vitamin D and active omega 3 fatty acids or placebo omega 3 fatty acids. If we place these options into a grid format, we can easily see the four different groups and why it’s called two-by-two factorial design (Figure 1). To summarize, the participants either take both active supplements, one active and one placebo supplement, or the placebo form of both supplements.
| Active Omega 3 | Placebo Omega 3 | |
| Active Vitamin D | Active Vitamin D/Active Omega 3 | Active Vitamin D/Placebo Omega 3 |
| Placebo Vitamin D | Placebo Vitamin D/Active Omega 3 | Placebo Vitamin D/Placebo Omega 3 |
The replacement of the active supplement with a visually identical placebo is crucial to ensure that participants do not know if they are taking the active form or not; hence, this study is “placebo controlled.” Many clinical trials are conducted in this way, as it prevents the placebo effect, avoiding bias. The placebo effect occurs when a patient believes they are engaging in an activity, like taking a drug or supplement, that will theoretically make them feel better. Believing their health will improve is sometimes enough to alter the body’s mechanisms, leading to endorphin release and improving the patient’s health. It could also subconsciously or consciously change behavior. For example, a participant who thinks the supplement is making them healthier may be motivated to make lifestyle changes that lead to better health, but skew the study. Knowing whether a participant is taking the active or placebo form of a drug can also alter the behavior of the experimenter that interacts with the participant. For example, if a doctor knows a patient is taking an active form of a drug, the doctor may speak more positively about treatment. This reassuring attitude from the doctor then further affects the patient’s behavior and thinking. In this study, neither participant nor the experimenters who interacted with the participant knew which group the participant was in. This is called a “double blind” study, and it eliminates potential bias, generating more accurate data. In single blind studies, researchers are aware of the participants grouping, while participants remain in the dark.
The study is also described as randomized. This means that the participants in the study were randomly assigned to their group. This eliminates bias and gives data that is roughly representative when looking at certain characteristics present or not present across groups. For example, we should expect that the number of participants who have a family history of autoimmune disease is roughly equal in each of the four groups due to randomization.
Nationwide describes the setting; in this case, the setting was limited to anywhere in the United States. Settings are chosen not only based on their relevancy, but also on convenience. Because this study was examining autoimmune diseases, which are not limited based on location, the study was conducted nationwide. Studies may incorporate international data, but this is more complicated and difficult to conduct. A nationwide study allows for diversity within the participants. This is incredibly important to understand how certain factors (e.g., socioeconomic factors, sex, ethnicity, body mass index, family history of disease) may also affect the outcome of a trial.
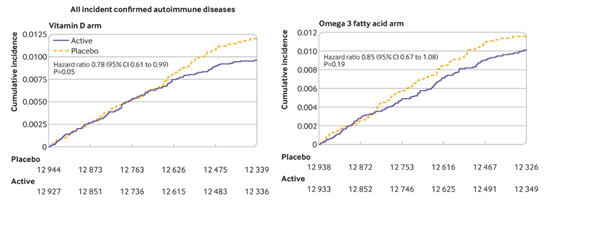
Overall, the study found that supplementation with vitamin D over five years with or without omega 3 supplementation reduced autoimmune disease development by 22%. Vitamin D supplementation was more effective when looking at participants who had a lower body mass index. A lower body mass index is correlated with less body fat; vitamin D is sequestered in fat cells, which would cause dilution of the supplement and therefore less fat may lead to a more potent dose of vitamin D. However, additional studies show that a higher supplementation for those with a higher body mass index does not completely equalize the two groups, suggesting more factors at play. Additionally, Omega 3 supplementation over five years with or without vitamin D supplementation reduced autoimmune development by 15%. While this number was not statistically significant overall, omega 3 supplementation did significantly reduce disease development when looking at participants with a family history of autoimmune disease. A summary of these results can be seen in Figure 2.
Understanding study parameters is important for avoiding bias while also including participants that are representative of the public. This study incorporated several different facets to avoid bias and to generate data that may be relevant for a diverse group of people.