Title: Biomimetic Total Synthesis of (±)-Griffipavixanthone via a Cationic Cycloaddition–Cyclization Cascade
Authors: Kyle D. Reichl, Michael J. Smith, Min K. Song, Richard P. Johnson, and John A. Porco Jr.
Journal: Journal of the American Chemical Society http://pubs.acs.org/doi/10.1021/jacs.7b09265
Year: 2017
Some microbes, fungi, and plants contain some of the most incredible chemical machines in charge of synthesizing complex secondary metabolites that allow their producer organisms to proliferate. These compounds broadly referred to by chemists as natural products, constitute a highly sought-after class of molecules because of their enticing architectural diversity and their interesting biological properties with potential applications in medicine and drug discovery. Even in today’s pharma era of highly streamlined synthesis and assaying of therapeutic candidates, natural products still prevail as our best source and inspiration for drugs and drug leads. Yet, isolation of these compounds from their biological source is often low-yielding and seldom allows for the desired purity needed for further research.
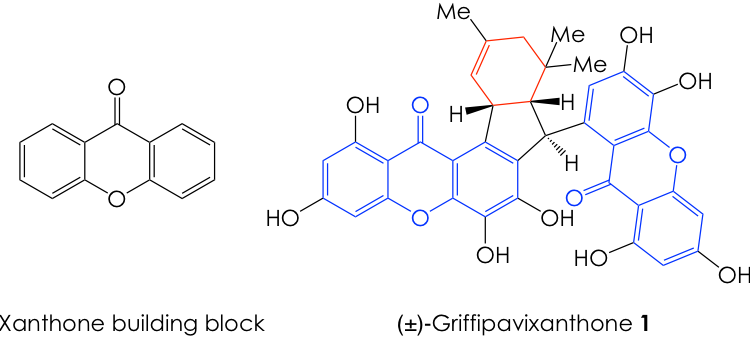
As one might expect, synthetic chemists have taken this drawback as an opportunity to deploy their creativity, using readily available starting materials in inventive, and often unorthodox, pathways towards an elegant total synthesis of a heavily functionalized natural product. However, sometimes the most efficient routes derive not from unconventional transformations, but rather from chemical maneuvers that mimic those used by cells. Such is the latest effort from John Porco’s lab at Boston University, which culminated in the first chemical synthesis of (±)-griffipavixanthone (1, Figure 1) by emulating the way in which enzymes build this molecule in biology. This dimeric xanthone was first isolated from the extracts of the edible plant Garcinia oblongifolia in 1998 and is known to possess antioxidant properties. More recently, this compound demonstrated anti-cancer activity by inducing apoptosis (programmed cell death) in several types of cancer cell lines, and thus garnered the attention of medicinal and synthetic chemists alike.

Given the sheer size and chemical complexity of 1, it is no wonder why it has remained an elusive total synthesis target. Nonetheless, the authors addressed this challenge by taking inspiration from nature. They recognized that, in its host plant, the cyclohexene core of 1 (Figure 1, in red) is formed via a cyclization reaction known as Diels-Alder cycloaddition (or [4+2] cycloaddition). This fundamental transformation occurs when the 4 pi-electrons (electrons in the double bonds) of a diene react with the 2 pi-electrons in a dienophile in a cyclic fashion to furnish a cyclohexene adduct (Figure 2A). Based on this knowledge, the Porco group envisioned a retrosynthetic pathway for 1, where they planned the synthesis backward, starting from the target molecule 1 and moving their way back to simpler intermediates and starting materials.

Their retrosynthesis involved two key steps. The first one is a Diels-Alder reaction between two quinone-derived building blocks, the diene 2 and the dienophile 3, to link the two quinone moieties and produce the dimeric adduct 4 with the desired cyclohexene core. The second key step yields 1 via an elegant cyclization of intermediate 4. What is even more impressive is the fact that a single quinone derivative, 5, can sequentially afford both the dienophile 3 and diene 2 via tandem oxidation and isomerization events (Figure 2B).
An arduous screening of several candidate Lewis and Brønsted acids to activate the diene, the details of which are beyond the scope of this post, led the group towards some runner-ups that not only enabled the Diels-Alder reaction to occur with the correct regio- and stereoselectivity, but also facilitated the subsequent arylation of the cycloadduct 4 that culminates in griffipavixanthone. They eventually settled for zinc iodide (ZnI2) as a Lewis acid or trifluoroacetic acid (TFA) as the Brønsted acid, both of which allowed them to go from building block 5 to the target 1 in a single-pot set of cascade reactions. Of course, this is just a simplified version of the story, as there are subtleties not covered here such protecting groups and small chemical tunings of the building blocks to improve their behavior, but the synthetic canvass over which the Porco Lab developed their proposal is pretty much set. I encourage you to take a look at the full article if you are curious about these details.
The present work sets up to conquer a challenge that very few in the world of Chemistry prefer to venture in, and it does so quite successfully, attaining the first total synthesis of griffipavixanthone in a deca-milligram scale, which, believe it or not, is actually a commendable result in the total synthesis world. While we are yet to see if this route is scalable enough to provide material for more thorough bioactivity studies, it is a laudable synthesis. This effort, while intimidating even to fellow chemists, is a beautiful exponent of intellectual aplomb, synthetic expertise, artistic taste, and humble recognition that sometimes enzymes do it best. Just as a composer combines notes and tunes their instruments to develop an impeccable symphony, the authors niftily combined creative, bio-inspired chemical tactics and meticulously tweaked steps to arrive at the target molecule as seamlessly as possible, proving once more that total synthesis has as much artistic skill as it does raw synthetic talent.