Guest post by Michael T. Yeung, postdoc at Northwestern University
Authors: Liyan Xie, Qing Zhu, Guozhen Zhang, Ke Ye, Chongwen Zou, Oleg V. Prezhdo, Zhaowu Wang,Yi Luo, and Jun Jiang
Journal: Journal of the American Chemical Society
Year: 2020
Doping is an important process in the electronics industry that controls the conductivity of a semiconductor by introducing foreign atoms into a crystalline lattice. Materials used in the electronics industry like silicon must be fabricated with these impurities so that the flow of electrons within them can be precisely controlled. These dopants then impart an “electrical imbalance” making the semiconductor more positive of negative; these excess charges help carry current.
Typically, the dopant must be incorporated at high temperatures, and these harsh conditions can lead to structural rearrangement of the semiconductor or even reaction with the environment. Compounding this is the fact that many potential dopants are volatile at the temperature of synthesis. For examples, let’s consider silicon, which is found in most processors. The variety of p-type dopants that are possible for silicon are boron, aluminum, gallium, and indium as they are trivalent and contain one less electron than silicon. This in turn creates “holes” or electron-deficiencies which help carry current (a more detailed guide can be found here) But, because indium is volatile, it has not found as many applications in favor of the easier to prepare materials. This can be a problem as many of the most interesting solid-state compounds require exotic dopants, and synthetic limitations preclude their study.
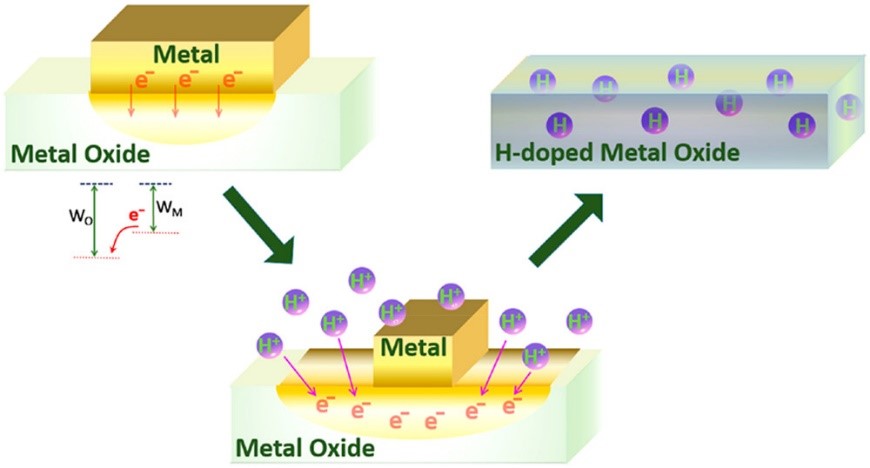
In their recent paper, Xie et al demonstrate that simply stirring metal oxides with metals under acidic conditions at ambient temperature can lead to hydrogen doped semiconductors. Normally, the addition of acids to oxides results in their dissolution, but the novelty of this new technique comes from introducing reducible metals in solution, so that the acid reacts with the metal instead of the oxide. The fundamental principle comes from the matching of work function (the amount of energy needed to remove an electron to vacuum) of both the reducing metal and the metal oxide, which encourages the diffusion of hydrogen into the lattice into the voids around the metal cation and oxygen anion (Figure 1). Perhaps the most striking effect from the insertion of hydrogen is the prominent shift in color of the powders from white to blue (Figure 2). As all color comes from electronic transitions, this color change comes from a change in valence corresponding with the incorporation of an electron.

These results show many other dopants can possibly be incorporated by this soft chemical approach, with potential applications in photocatalysts and electronics. Moreover, the gentle conditions afforded with this solution-based method avoids the need of equipment such as furnaces and opens up a new field of gentle doping without decomposition.
