Title: It is Better with Salt: Aqueous Ring-Opening Metathesis Polymerization at Neutral pH
Authors: Jeffrey C. Foster, Marcus C. Grocott, Lucy A. Arkinstall, Spyridon Varlas, McKenna J. Redding, Scott M. Grayson, and Rachel K. O’Reilly
Year: 2020
Journal: Journal of the American Chemical Society, 142, 32, 13878-13885
My organic chemistry professor always impressed upon us that the golden ideal of organic chemistry is to perform reactions that create bonds between two carbon atoms. Unfortunately, despite organic chemistry being quite literally the study of carbon and the molecules it forms a part of, the organic chemists’ toolbox for making these carbon-carbon bonds is limited. In many cases these reactions must be performed under harsh conditions using very environmentally unfriendly chemicals. (And here you were probably thinking that anything with the word organic in it had to be good for the environment. This is not your local farmer’s market.)
As the field of chemistry has progressed, so has our options for performing chemical reactions in an environmentally responsible way. The authors of this paper recognized the importance of practicing chemistry in an environmentally responsible way, and set about investigating a ring opening metathesis polymerization reaction, or ROMP , that can take place in water. ROMP reactions are just as fun as they sound, at least if you enjoy organic chemistry . They lead to the creation of polymers, the long chains of linked-together carbon-based molecules that make up anything from the fibers of your clothes to the plastic containers you use to store food.
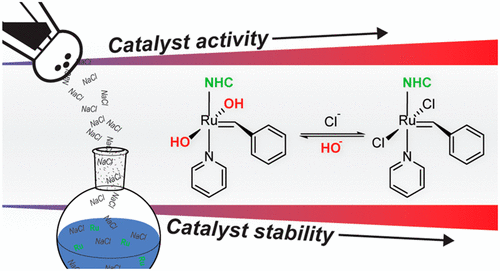
Illustration of the ruthenium catalyst used in this paper, from JACS (J. Foster et al, J. Am. Chem. Soc., 2020, 142, 32, 13878-13885.)
So what makes this ROMP special? First off, it takes place in what the authors refer to as an aqueous media, which just means water is being used to dissolve everything. Most organic chemistry doesn’t use water to dissolve things because most organic compounds are hydrophobic and won’t dissolve in water at all. Water is a great solvent to use because unlike most common organic solvents it doesn’t need any special safety precautions or waste disposal procedures. You can just pour it down the drain (assuming there are no other harmful chemicals present), no harm done!
Another factor that makes this reaction unique is that it can be performed at a neutral pH, without having to add an acid or a base to the water. Initially the researchers believed that it was necessary to add hydrochloric acid to the reaction and therefore lower the pH. However, an attempt to use a different acid revealed to the researchers that the importance of adding hydrochloric acid, which separates into a positively charged hydrogen ion and a negatively charged chloride ion when dissolved in water, was actually the formation of the chloride ion. The authors were able to substitute the hydrochloric acid with other, pH-neutral sources of chloride ions like common table salt, NaCl, and successfully create the desired polymers without the need to use a dangerous strong acid like HCl.
The addition of these chloride sources had another happy side effect – the ions were able to make the ruthenium catalyst that enables the ROMP reaction to work more efficiently. This meant that the researchers could use a smaller amount of the catalyst, which is another win for the environment as this catalyst is not eco-friendly.
The work to find more eco-friendly ways of making important chemical compounds continues, but this article represents a promising step forward. In the future, chemists who work with this kind of polymer-forming reaction now have new options to do their chemistry under greener conditions, without the need for traditional toxic organic solvents or harsh, strong acids.