Title: Identification of Novel Antifreeze Peptides from Takifugu obscurus Skin and Molecular Mechanism in Inhibiting Ice Crystal Growth
Authors: Fujia Yang, Wenting Jiang, Xu Chen, Xuan Chen, Jinhong Wu, Jianlian Huang, Xixi Cai*, and Shaoyun Wang*
Journal: Journal of Agricultural and Food Chemistry
Year: 2022
DOI: https://doi.org/10.1021/acs.jafc.2c04393
Featured image by Bozhin Karaivanov
In the agricultural industry, techniques for food preservation are critical for maintaining food quality and structural integrity. Cryopreservation is one such technique that is used to maintain food quality. Cryopreservation or specifically, cryogenic freezing involves the use of cryogens such as liquid nitrogen to freeze food products. This allows industries to preserve the freshness, quality and safety of their foods while minimizing costs for food maintenance.
While cryogenic freezing is a common and effective technique, it is limited by presence of ice crystal formation which lowers quality of frozen food. Quick-freezing food at cryogenic temperatures ensures that smaller, more evenly distributed ice crystals form which improves the quality and taste of frozen food products. Though the impact of ice crystals can be mitigated during the cryogenic freezing process, ice formation is still a significant threat to food quality during low temperature transportation of the frozen products. Anti-freeze peptides are one way to address this problem.
Anti-freeze peptides are implemented during the transportation process to protect food from sub-zero environments by reducing the freezing point of solution without greatly affecting the melting point. This is known as thermal hysteresis. In terms of ice crystal formation, the antifreeze peptides manage and restrict ice formation that can severely damage foods.
The development of anti-freeze proteins and peptides are critical for the food industry to protect and maintain not just foods but other organisms as well. There is growing interest in these developments as the anti-freeze peptides are effective anti-freeze agents, have stable properties and low productions costs.
Recent research by Fujia Yang and colleagues has identified novel antifreeze peptides from the skin of an Obscure Pufferfish (Takifugu obsurus). The skin of this animal is rich in collagen which provides good source for anti-freeze peptides. In the study, Yang and colleagues acquired these novel antifreeze peptides sequences to study their molecular mechanisms of action with implications towards development of future efficient cryopreservation agents.
The researchers isolated, purified, and examined the anti-freeze activity peptides to identify which peptides had the best anti-freeze properties. The various anti-freeze peptides that were extracted were first isolated and separated by ion exchange chromatography. Those with the highest anti-freeze activity were then further separated by gel-filtration chromatography and subsequently characterized by liquid phase mass spectrometry (LC-MS/MS) to identify peptides with the highest anti-freeze properties (Figure 1). The degree of each peptide’s anti-freeze ability was evaluated by testing the viability of peptide-dosed Streptococcus thermophiles after freezing.
Table 1. Data on peptide sequence, hydrophilicity, molecular weight and source of novel antifreeze peptides. Figure is adapted from Yang et al., 2022.
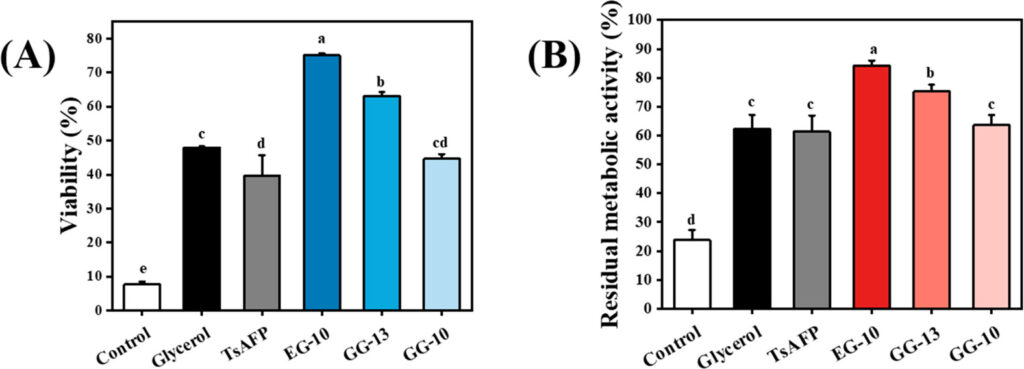
The viability study in Figure 1A shows that peptide EG-10 have greatest viability of the novel peptides after freezing stress. Figure 1B shows that EG-10 also has the greatest impact on residual metabolic activity relative to the other peptides. The impact of higher metabolic activity is significant because freezing stress (i.e. ice crystals) can induce damage towards the cell membrane and enzymes resulting in lower cellular metabolic activity. Therefore, a higher residual metabolic activity and viability are indicative of higher anti-freeze activity.
To evaluate the structure and overall molecular mechanisms of action, molecular docking simulations and other traditional techniques modelled how the anti-freeze peptides interacted with ice crystals. The authors stated that there are no current methods to study the molecular mechanisms between antifreeze peptides and ice crystals, however molecular simulation-based research has generated a lot of interest. The authors utilized molecular docking which looks at the interactions of molecules using computer technology (e.g. simulations, programming) and statistics.
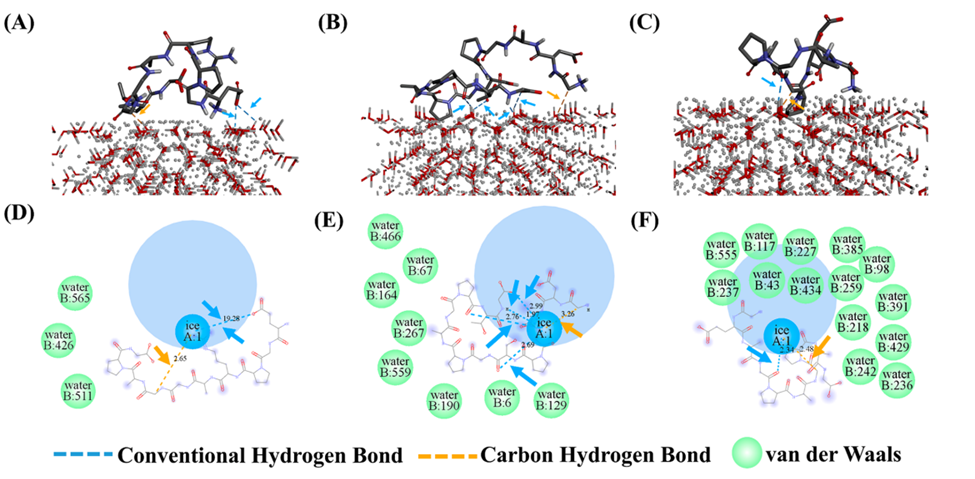
Yang and colleagues modelled the three novel antifreeze peptide sequences with a prediction modeling program and investigated the interaction of the sequences and ice crystals through molecular docking. This allowed them to determine the final configuration of the interactions between the antifreeze peptides with ice crystals (Figure 2). The main take away from this figure is that it looks at the binding energy of peptides with ice crystals, where the authors evaluated the binding total energy of the system, which provides an indication on binding stability. The greater the absolute value of total energy, the more stable the binding. The study demonstrates that EG-10 peptide had best cryoprotective effect because it has lower absolute total energy, and fewer hydrogen bonds and nonbond interactions with ice crystals. The study argues that this may be attributed to the unique α-helix structure of EG-10 (unlike the other 2 peptides), that provide greater stability for interaction with ice crystals. This indicates that during the interaction, EG-10 will not change its structural conformation when binding with ice crystals hence demonstrating stronger cytoprotectively than the other 2 novel peptides.
Overall, Yang and colleagues have identified novel antifreeze peptides, and were the first to analyzed molecular interactions between peptides and ice crystals using molecular docking techniques. Their findings have implications for food and agricultural industries.

