Featured image reprinted (adapted) with permission from Pixabay.
| Title: | High-affinity adsorption leads to molecularly ordered interfaces on TiO2 in air and solution |
| Authors: | Jan Balajka, Melissa A. Hines, William J. I. DeBenedetti, Mojmir Komora, Jiri Pavelec, Michael Schmid, Ulrike Diebold |
| Publication Info: | Science 24 Aug 2018: Vol. 361, Issue 6404, pp. 786-789 |
You might recognize titanium dioxide as a common ingredient in the most uncool kind of sunscreen – the kind that leaves you greasy and white all over. But it’s also a common material in photocatalysts, which can use sunlight to drive chemical reactions. In fact, titanium dioxide (or TiO2) was used in the seminal paper demonstrating water splitting (the Holy Grail of photocatalysis).
Scientists have known about the self-cleaning properties of titanium dioxide for a while, too. These self-cleaning properties mean that organic molecules – like grease or soot – adsorbed (or stuck) onto the surface of titanium dioxide will break down in sunlight. Titanium dioxide can also be hydrophilic, which means that rain will wash away any dust or dirt that collects on a coating of this material.

But scientists have also found that titanium dioxide can be hydrophobic – meaning it repels water, the opposite of hydrophilic. Titanium dioxide is only hydrophobic under certain circumstances: specifically, when the surface is not being illuminated with ultraviolet light. This means that a chemical change is occurring on the surface of the titanium dioxide – and any changes in the molecular structure at the surface could affect photocatalysis using titanium dioxide, so they’re important to understand. Previous reports on the surface have been contradictory, with explanations ranging from adsorbed H2O [1] to various adsorbed organic compounds present in air.[2,3]
To study the hydrophilic/hydrophobic transition, these researchers took data on the molecular structure of a coating of titanium dioxide using STM and XPS, types of microscopy that can show chemical changes at a surface. They exposed titanium dioxide to a drop of water, first under ultrapure conditions and then in ambient air, and looked at how the surface changed.
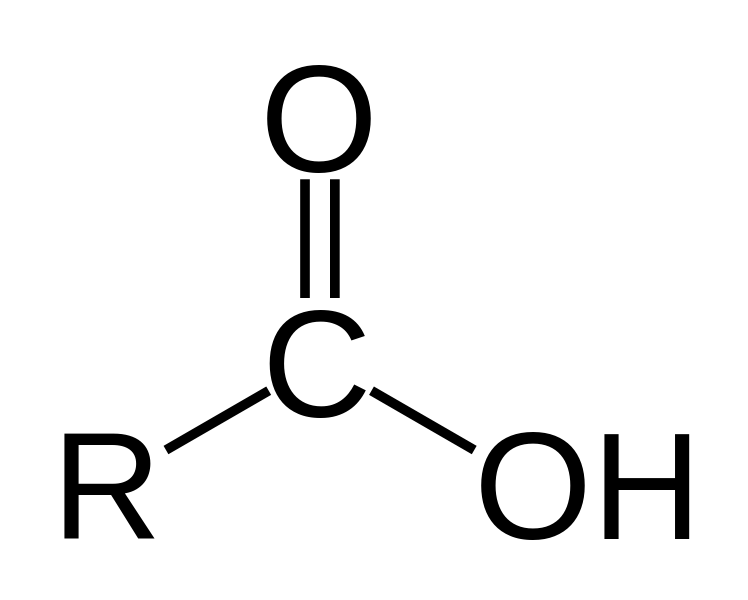
The titanium dioxide exposed to ultrapure water showed virtually no change in its molecular composition. However, when exposed to water under ambient air, carboxylic acids adsorbed to the surface to form a thin layer of hydrophobic molecules.
This research sheds some light onto the mystery of hydrophilic/hydrophobic titanium dioxide. Understanding the adsorption of different chemicals onto photocatalytic materials like titanium dioxide may not seem very important for sunscreen – but it certainly matters for applications in photocatalysis, where surface catalytic sites need to be exposed to work effectively.
[1]
G. Serrano et al. Molecular ordering at the interface between liquid water and rutile TiO2(110). Adv. Mater. Interfaces 2, 1500246 (2015). doi:10.1002/admi.201500246
[2]
H. Hussain et al. Structure of a model TiO2 photocatalytic interface. Nat. Mater. 16, 461–466 (2017). doi:10.1038/nmat4793pmid:27842073.
[3]
A. Song et al. Nanoscale solvation leads to spontaneous formation of a bicarbonate monolayer on rutile (110) under ambient conditions: Implications for CO2 photoreduction. J. Phys. Chem. C 120, 9326–9333 (2016). doi:10.1021/acs.jpcc.6b02132

