Title: Food for thought
Authors: Bruce C. Gibb
Journal: Nature Chemistry (2020)
Diet fads run amuck in the information-saturation era we live in. It is hard to know what you can really trust. Clearly, eating patterns that are backed by high quality research are the ones to follow, but the scientific literature can be difficult to digest. Studies can be misinterpreted and over-hyped or over-scrutinized by the media.
Most people have heard of intermittent fasting but could never imagine “starving” themselves, even just for a few extra hours each day. Let’s briefly dive into the chemistry behind how intermittent fasting works and why it’s a useful evolutionary function that everyone can take advantage of.
The problem of overeating does not just affect humans at the level of the individual. Between 1962 and 2002, food consumption rose by 25% while sedentary lifestyles increased. A large contribution comes from the huge rise in the consumption of fast-food, especially in the United States, which rose overall 3-fold in the same period. These trends bring to the foreground global issues including concerns over food supply and the general health of the world population.
The act of fasting goes back many centuries and is an important aspect of many religious ceremonies. Humans have at least indirectly benefited for thousands of years so why don’t more people take advantage of it? It could be a lack of understanding or an addiction to sugar-packed foods. The first one we can solve, the second is not so easy. What does modern science tell us?
Many are familiar with the key study in rats that showed considerable healthy benefits from reduced caloric intake and eating at certain times of day. These results have been recapitulated time and time again in a multitude of organisms – from protozoans and spiders to fish and chickens. Although studies have been performed in humans, there are many variables still to consider like the timing of the fast and what you should eat during the eating periods. Further research, especially longitudinal (across a lifetime) studies, is still necessary but the prospects look extremely promising.
Flipping the Switch
What’s really important to understand is that our body has TWO primary ways to generate ATP, the energy-carrying molecule that powers all of life. Most people are familiar with the first, glycolysis (breaking down sugar) – it is the immediate energy boost we get from munching down a candy bar. The second method is somewhat counterintuitive and comes when we are either fasting or in a state of eating only protein and fat as fuel sources.
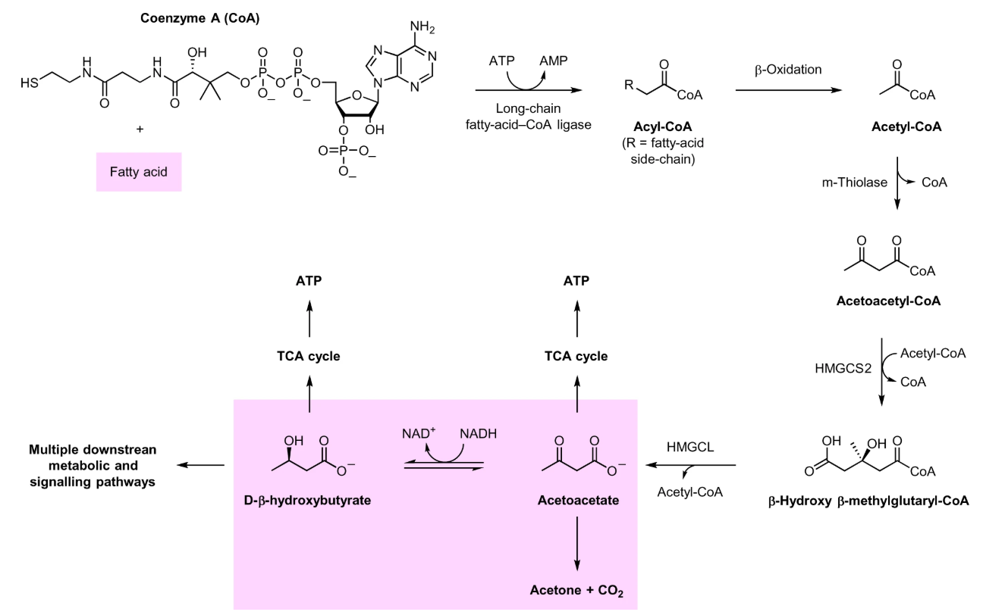
During fasting, cells activate special pathways that have a ton of benefits. Research suggests that fasting also results in larger epigenetic changes that can be passed on to an individual’s offspring. The secret comes from ketone bodies. The chemistry is just one simple step – the conversion of fatty acids into beta-oxidation products. However, this pathway is only prioritized when major sugar sources are not present in the body. Seen in Figure 1, this is the step from acetoacetyl-CoA through enzyme HGMCS2.
The research picture for intermittent fasting in humans is just coming into view, though preliminary studies look promising. The health implications are exciting, and the basic biochemistry is sound. Our bodies evolved two primary systems to generate energy so why not use both?