Authors: Haytham O. Tawfik, Mohamed M. Saleh, Andrea Ammara, Eman F. Khaleel, Rehab Badi, Yomna T. T. Khater, Rabab A. Rasheed, Ahmed A. Attia, Salma M. Hefny, Eslam B. Elkaeed, Alessio Nocentini, Claudiu T. Supuran, Wagdy M. Eldehna, and Moataz A. Shaldam
Journal: ACS Journal of Medicinal Chemistry
Year: 2024
Header image reprinted from Tawfik et al.
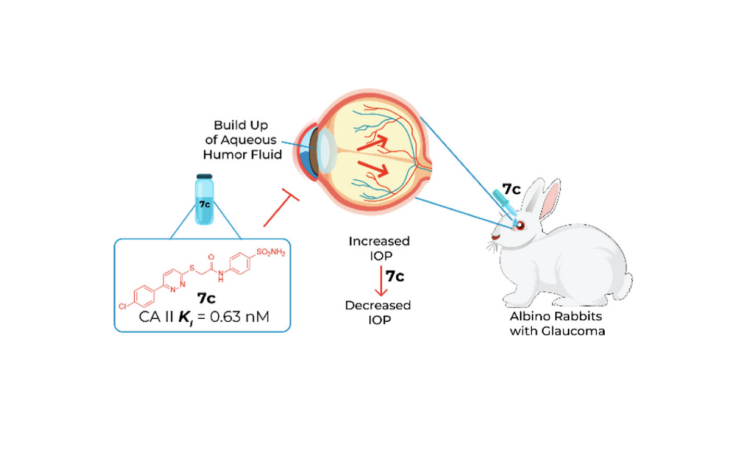
Glaucoma is a progressive neuropathic condition characterized by a drainage malfunction in the eye. This involves excessive fluid build up, elevated intraocular pressure (IOP), and subsequent damage to the optic nerve. This nerve transmits electrical signals from the eyes to the brain. When damaged, patients experience gradual or sudden vision loss with a risk of permanent blindness. While the condition affects millions of individuals worldwide, symptoms are not always apparent, especially in the early stages of the disease. Thus, glaucoma is oftentimes referred to as the “silent thief of sight.” Fortunately, a variety of treatment methods exist including medication, laser therapy, and surgery.
Medication is the least invasive treatment option but is often accompanied by undesired side effects. The primary goal of these medications is to lower the IOP by slowing aqueous humor (fluid) production in the eye. A metalloenzyme called carbonic anhydrase (CA) is responsible for indirectly catalyzing this fluid production. Thus, by inhibiting this enzyme with carbonic anhydrase inhibitors (CAIs), the IOP can be lowered.
While some CAIs exist and are used for the treatment of glaucoma, many produce a wide range of side effects because they interact with multiple CA isoforms. It turns out that humans express 16 different isoforms of this enzyme, and they’re found in various tissues and organs! Finding a way to selectively block the isoforms that are related to glaucoma (such as CAII) is ideal because it will slow the secretion of aqueous humour with minimal side effects. Tawfik et al. recently embarked on a medicinal chemistry campaign to design and test novel compounds that can treat glaucoma with enhanced selectivity and potency.
The researchers synthesized a series of compounds containing a unique phenyl-pyridazine moiety (2 rings connected; one of them containing 2 adjacent nitrogens). This motif has not been extensively explored for anti-glaucoma drug development. They varied the length of the carbon linker and also modified the substituent off the phenyl ring to explore how changing the compounds’ electronic properties and lipophilicity affects their biological activities (figure 1). Using a robust and in vitro stopped-flow CO2 hydrase assay, the group was able to determine the potency of each analogue through their respective Ki values. In short, a lower the Ki value means higher potency because less compound is needed to achieve the desired inhibitory effect on the CAII enzyme.
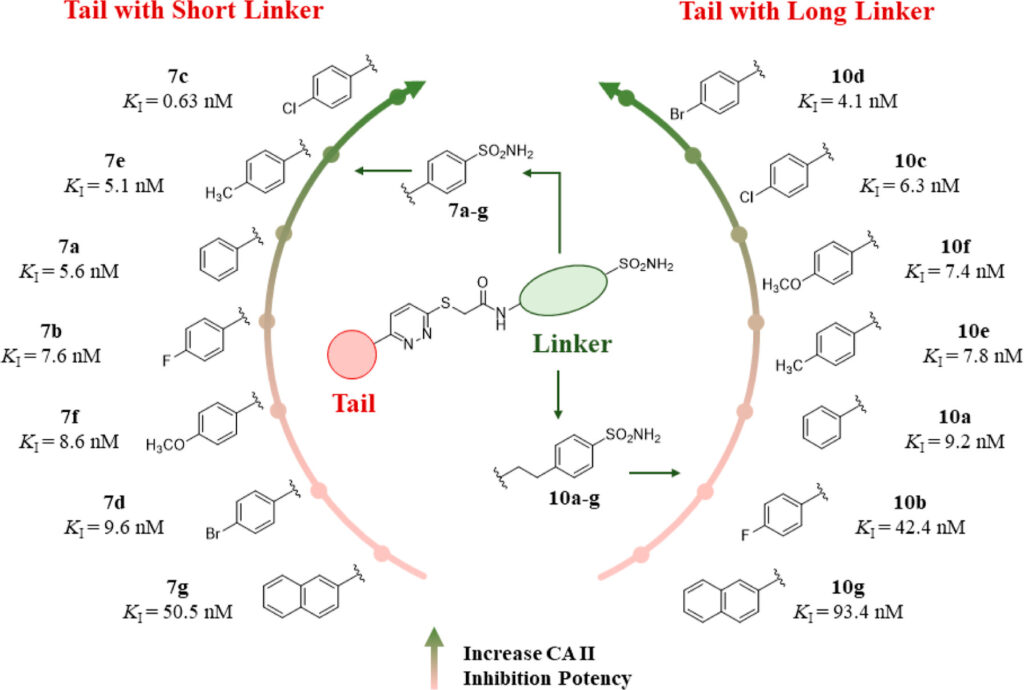
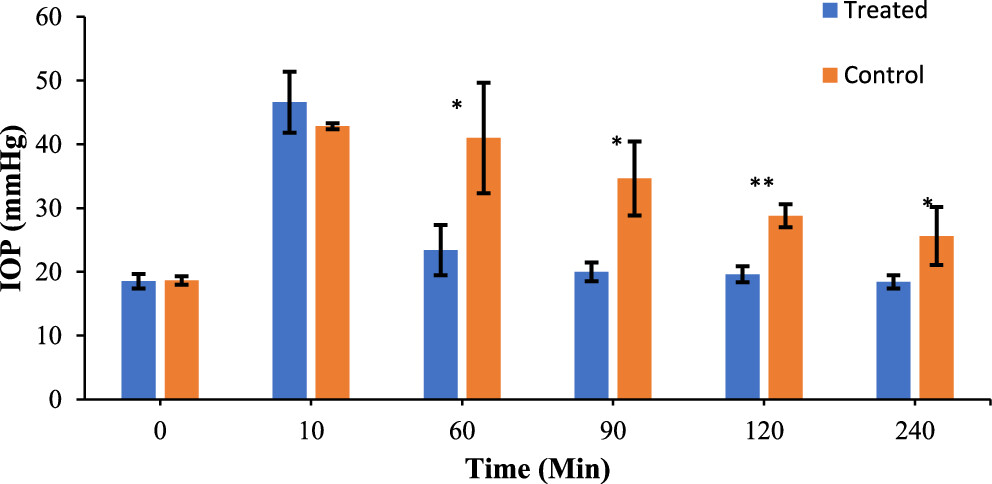
As shown above, derivative 7c performed the best and exhibited subnanomolar activity against CA II with a Ki of 0.63 nM. In addition, all the compounds were found to prefer the CAII isoform over the CAI one. In fact, they were all more selective on CAII than acetazolamide, an anti-glaucoma drug used in clinical settings. To test how well compound 7c performs in vivo, the researchers employed rabbit models with ocular hypertension. They measured their IOP at time 0 on the graph below just before glaucoma induction, which was achieved by the intravitreal injection of a NaCl solution (figure 2). They subsequently administered compound 7c in the rabbits’ right eyes and vehicle control in the left. The IOP values were measured using a Schiotz (indentation) tonometer over the course of 240 minutes. There is a statistically significant difference between the IOP of the eyes treated with 7c and those treated with the control. This difference is prominent after just 1 hour and is maintained for 240 minutes.
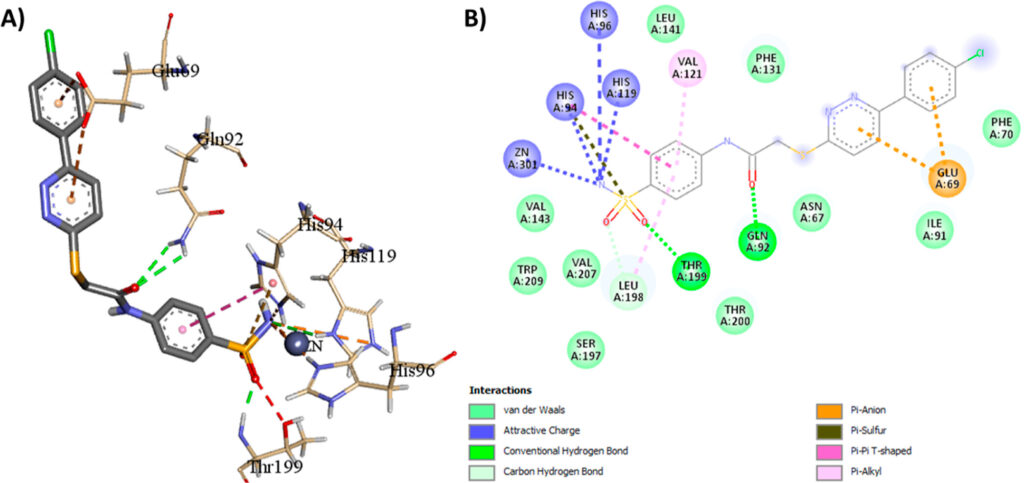
To better understand why compound 7c performed so well and was highly selective for the CAII isoform over other isoforms, the researchers performed a molecular docking study. This revealed important binding interactions between 7c and the CAII enzyme binding pocket (figure 3). The key binding mode that enables the high preference for the CAII receptor is the anion-pi interaction (orange) between the phenyl-pyridazine moiety and the Glu69 residue in the rim region of the active site. This portion of the enzyme is non-conserved among the CA isoforms and can be exploited for selectivity tuning. This interaction was observed in addition to the standard binding pattern of sulfonamide CAIs.
Overall, the researchers successfully synthesized a series of phenyl-pyridazine-based sulfonamides and tested their inhibitory properties against the CAII enzyme. All compounds were found to be highly selective, with compound 7c being the most potent with subnanomolar activity. Hopefully, we’ll see new medications enter the clinic for the treatment of glaucoma with minimal undesired side effects.
~~~
This is an unofficial adaptation of an article that appeared in an ACS Journal of Medicinal Chemistry publication. The ACS has not endorsed the content of this adaptation or the context of its use.
Figures are adapted with permission from Tawfik et al. Copyright 2023 American Chemical Society.
Full Reference: Tawfik, H. O.; Saleh, M. M.; Ammara, A.; Khaleel, E. F.; Badi, R.; Khater, Y. T.; Rasheed, R. A.; Attia, A. A.; Hefny, S. M.; Elkaeed, E. B.; Nocentini, A.; Supuran, C. T.; Eldehna, W. M.; Shaldam, M. A. Discovery of Novel Pyridazine-Tethered Sulfonamides as Carbonic Anhydrase II Inhibitors for the Management of Glaucoma. Journal of Medicinal Chemistry 2024. DOI:10.1021/acs.jmedchem.3c02279.