| Paper | Amphiphilic dendrons as supramolecular holdase chaperones |
| Authors | Elizabeth R. Piedmont, Erin E. Christensen, Todd D. Krauss, Benjamin E. Partridge |
| Journal | RSC Chemical Biology |
| Year | 2023 |
| URL | https://pubs.rsc.org/en/content/articlelanding/2023/cb/d3cb00086a |
Our body produces proteins in a specific shape and size to perform their function, like exquisitely folded origami. If the protein loses its shape, it can also lose its ability to function properly, and may clump together with other misfolded proteins. This process is called aggregation, and the proteinaceous clumps are implicated as the causative agents of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s.
Our body can get rid of misbehaving proteins by various means, including molecular chaperones. These compounds assist new proteins by helping them fold correctly, move to the correct location in the cell, and even mark bad quality proteins for degradation. You can imagine that these molecular chaperones work similarly to chaperones at a school dance; they ensure that all the kids are where they should be, help resolve clothing mishaps, and break up disruptive fights – perhaps even better than Derry Girls’ Sister Michael.
There are many kinds of molecular chaperones in all shapes and sizes, such as dendrimers. Dendrimers are bush-like polymer chemicals made up of repeating “branches” called dendrons (Fig 1). We don’t produce dendrimers naturally, but synthetic polymers have been used to deliver various natural remedies to the body due to their ability to hold compounds in the cavernous space between the branches. Scientists at the University of Rochester wondered if dendrons could provide a new drug development avenue to prevent protein aggregation, and subsequent neurodegeneration, in Alzheimer’s disease.
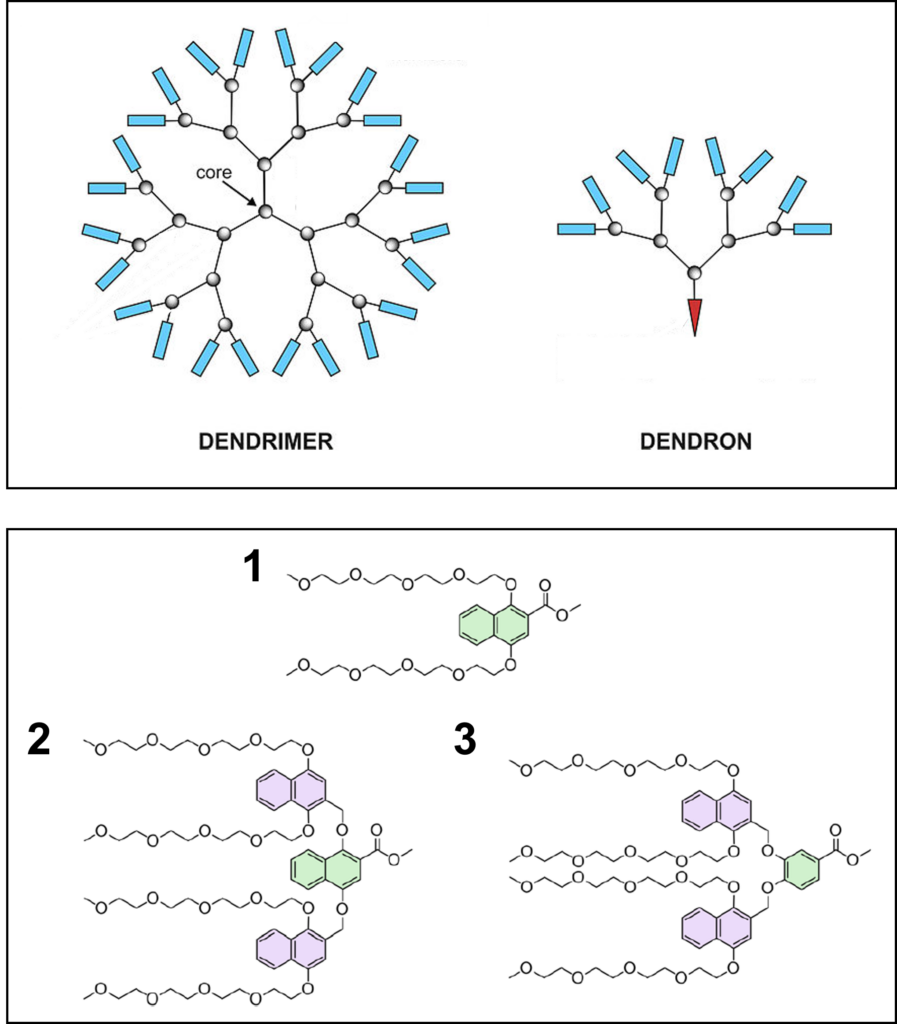
The scientists Piedmont et al. designed three dendrons around natural chaperones that use hydrophobic or water-hating properties to capture their protein aggregates. They showed that two of their three dendrons (#1 and #3 in Fig 1) dissolved well in water, spontaneously formed hollow spheres in a mechanism called supramolecular assembly, and did so with hydrophobic cavities. That meant their dendrons possessed all the initial desired qualities to capture and cage the aggregating protein. The chemists then mixed their dendrons 1 and 3 with Alzheimer’s disease protein Aβ, specifically a fragment containing amino acids 16 through 22 that form the hydrophobic core of Aβ aggregates (Aβ16-22).
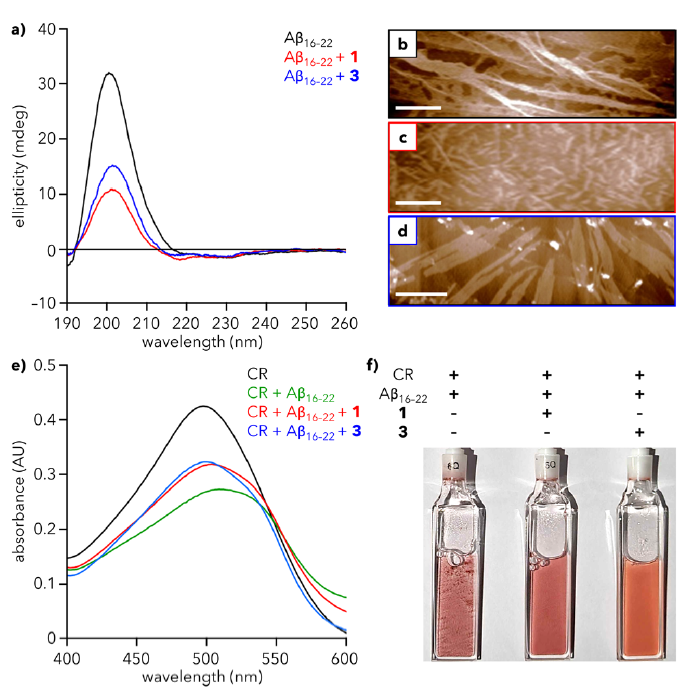
Their results from three separate tests showed that the presence of dendrons 1 and 3 reduced the quantity of Aβ aggregates or fibers formed by about half. First, the scientists looked at the impact of dendrons to Aβ’s secondary structure. Past experiments have shown that Aβ forms β-sheet structures in their aggregative state, which gives a distinct spectrum in circular dichroism assays (Fig 2A). The peak at 200 nm in black (Aβ alone) is decreased by half when the dendrons are present (blue and red). Observing the Aβ fibers under atomic force microscopy, the scientists saw that the fibers shortened and changed morphology when in the presence of the dendrons (Fig 2B/2C/2D). Additionally, the average particle height was increased about 4 times, which is a positive indicator that the dendrons are assembling around the Aβ peptides and capturing them. Piedmont et al. also used a Congo Red assay, a color changing compound that reacts to hydrophobic proteins, to monitor the aggregation more directly. Dendrons 1 and 3 reduced the total aggregation observed, as indicated by the increase in absorbance of Congo Red (Fig 2E). Finally, the turbidity or cloudiness of a sample is a physical indicator of protein aggregation. The Aβ samples visually showed a clearer liquid in the presence of dendrons 1 and 3, as shown in Fig 2F.
Dendrons provide some additional benefits important for drug development in comparison to other chemicals. While many drugs have a difficult time crossing the barrier between your bloodstream and your brain, dendrimers and their individual dendron branches show promise in reaching the target area. Therefore, the drug may stay as branches until it encounters the protein target, then perform an “Avengers Assemble!” maneuver and surround the protein with the supramolecular assembly, preventing damaging aggregation. Though no aggregation is ideal, different fibers encapsulated by chaperone molecules may be easier for our immune system to remove from our brains and ultimately reduce neurodegeneration. Most importantly, the synthetic creation of these dendrons is straightforward and provides very pure, high-quality chemicals that may be streamlined for industry settings. Piedmont et al.’s results for the utility of dendrons as protein chaperones provide an encouraging new avenue for drug development in Alzheimer’s and other neurodegenerative diseases.

