Title: Hydrogen peroxide serves as pivotal fountainhead for aerosol aqueous sulfate formation from a global perspective.
Authors: Jie Gao, Haoqi Wang, Wenqi Liu, Han Xu, Yuting Wei, Xiao Tian, Yinchang Feng, Shaojie Song & Guoliang Shi
Journal: Nature Communications
Year: 2024
Many fossil fuels contain small amounts of sulfur which enters the atmosphere as sulfur dioxide (SO2) when these fuels are burned. Once in the air, this SO2 can oxidise to form aqueous-phase sulfate, either dissolved in cloud droplets or small particles suspended in the air (known as aerosols). Sulfate increases the acidity of rain particles, which has important environmental impacts, such as harming aquatic life and trees. Sulfate aerosol also reflects incoming solar radiation, which can result in a cooling of the Earth’s climate.
Legislation passed by governments around the world to limit emissions of SO2 has meant that recent decades have seen declines in atmospheric concentrations of both SO2 and sulfate. However, the declines in sulfate have not been as rapid as the declines in gas-phase SO2 indicating that increases in the rate of SO2 oxidation have counteracted the reductions in SO2 concentrations. There are many factors that must be accounted for as the atmospheric oxidation of SO2 is a complex process with many different potential pathways, many of which involve heterogeneous chemistry between gas and particle-phase species.
In their recent publication in Nature Communications, Gao et al. aim to probe the importance of different sulfate aerosol formation pathways using data from a global atmospheric chemistry model. The work investigates the relative importance of four different sulfate formation pathways involving the oxidation of SO2 by: hydrogen peroxide (H2O2), nitrogen dioxide (NO2), ozone (O3), or transition metal ions (TMI). The rates of each of these processes are sensitive to a wide range of physical properties, however one of the key parameters is aerosol acidity which can change as a result of the constituent compounds. For example, increased sulfate concentrations result in more acidic particles. The H2O2 pathway is relatively insensitive to changes in pH, whereas each of the other pathways show strong responses to changes in pH. The NO2 and O3 pathways are most prevalent in higher-pH (more alkaline) aerosol, whereas the TMI pathway is enhanced in acidic aerosol.
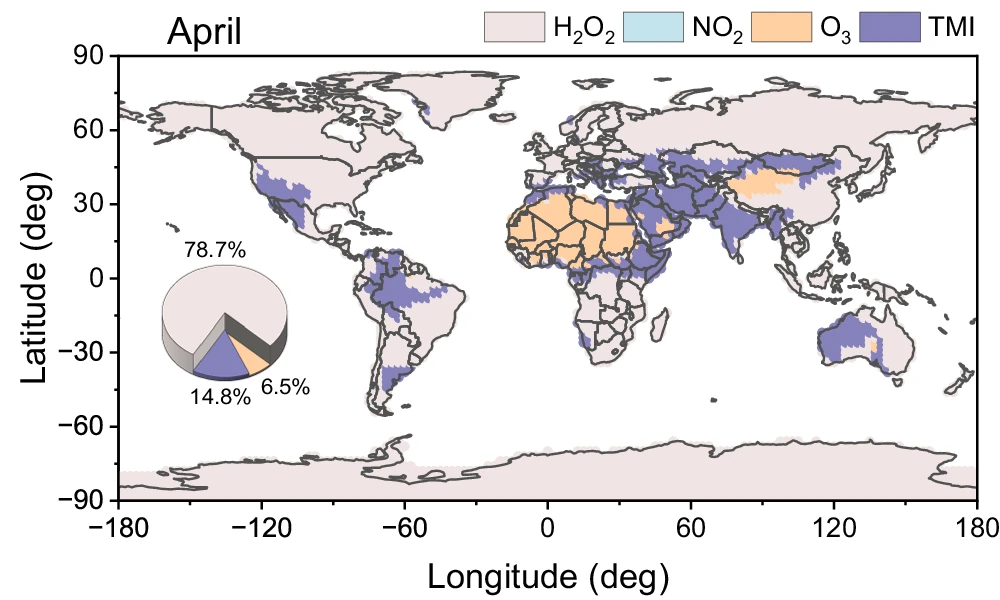
Through calculations based on results from a global atmospheric chemistry model, the researchers demonstrated that oxidation by H2O2 was the dominant aerosol sulfate formation pathway in most global regions. However, the TMI pathway was important in areas with high aerosol acidity. While the NO2 pathway only played a small role in sulfate formation in most environments, the O3 pathway was most important over desert regions such as the Sahara due to the high aerosol alkalinity over these regions.
Given the importance of aerosol pH for the formation of sulfate, the researchers note that the contribution of each of these pathways could shift as changes to human pollutant emissions impact aerosol acidity. For example, reductions in acidic aerosol components such as nitrogen oxides (NOx) could increase aerosol alkalinity and increase the importance of the O3 pathway outside of desert regions. Projected increases in ammonia (NH3) emissions could also increase the alkalinity of atmospheric aerosol.
A consideration of these oxidation processes is vital to properly avoid the negative impacts of atmospheric sulfate, beyond simply reducing SO2 emissions. For example, Gao et al. note that increases in H2O2 concentrations in Europe and North America have counteracted the recent decreases in SO2, slowing the decrease in sulfate concentrations. Finally, given the importance of H2O2 in sulfate formation, the researchers note that there is still lots of uncertainty around the formation processes of H2O2 in the atmosphere warranting further research.
Featured image by jiawei cui on Pexels.

