DOI: 10.1021/ja2108799
Author: Yihong Chen and Matthew W. Kanan
Journal: Journal of the American Chemical Society
Affiliation: Department of Chemistry, Stanford University, Stanford, California, United States
Take-home Importance According to the Authors: In aqueous NaHCO3 solution saturated with CO2, a Sn electrode with a native SnOx layer exhibited potential dependent CO2 reduction activity. In contrast, an electrode etched to expose fresh Sn0 surface exhibited higher overall current densities but almost exclusive H2 evolution over the entire 0.5 V range of potentials examined. Subsequently, a thin-film catalyst was prepared by simultaneous electrodeposition of Sn0 and SnOx on a Ti electrode. Metal/metal oxide composite materials are promising catalysts for sustainable fuel synthesis.
Take-home Importance According to the Blogger: An article on very fundamental science. In chemistry, many experiments come down to how can you be absolutely sure the observation corresponds with the variable you are changing. To take it one step further, how can you be absolutely sure the variable you are changing is having a significant effect on the system that will predict observations. So two things for your science: insight and careful experimentation.
Tidbit from the Blogger: At the very beginning of the blogging process, I thought maybe a small amount of personal opinions can add some flavors to a standard paper summary. Well, THIS is a blog. As much as I want to be objective here, I itch to dish out some personal opinions. So bear with me, please ^_^
Anyways, after hearing Professor Whitesides giving a talk on entropy/enthalpy compensation in proteins, I had a new perspective on the roles of solvent molecules that we know have a significant role on reactions but often turn a blind eye on. To be honest, it is a fascinating subject that I would never pick up during a everyday browsing of journals. I did pick up the another article immediately since it is about graphene and it is in Science. I am sure in freshmen chemistry, you have all heard about the fascinating properties of water. So as a tribute to that, I will leave these two articles at your leisure. I feel like may be I should actually blog about them. Well, another time. (DOI 10.1073/pnas.1114107108 and 10.1126/science.1211694 )
On another note, I am sure other authors will be saying something about the upcoming graduate school visits and general preparation for graduate school. Not to spoil too much, but it really came down to find the place, people, and research you like. It is going to be hard for the next couple of years, so might as well settle in comfortably.
Now now I have diverted your attention long enough from what I actually want to talk about, so here we go. As my general impression, this article sounded rather unintentional at the beginning, but the careful characterization paved a road to interesting discovery.
Summary
Small-molecule chemistry is an important part of the upcoming energy revolution if fossil fuel runs out one day. For a carbon-neutral energy industry, where carbon source is being recycled, or for an environmental reason, such as decreasing sea water acidification, transformations of CO2 are involved. One of the clean chemical approaches is electrochemcial reduction of CO2, and fabrication-facile low-cost metal electrodes seem to be good candidates in the case of industrial scale-up. With a large overpotential of 0.86V, it is assumed that the initial electron transfer to form CO2– radical anion. However, reported values can vary and often quoted to be sensitive of conditions if not completely reproducible.
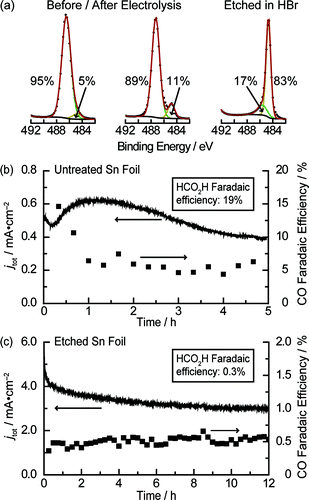
So a control experiment would be making the electrode surface absolutely pristine and check the chemical behavior. As shown below in the figures, by X-ray photoelectron spectroscopy (XPS) with chemical environment de-convolution, there are clearly two distinct types of binding motifs for Sn. Clearly from the measured Faradaic efficiency, the catalytic properties of the films are different, based on the composition of metallic Sn and oxides of Sn. With gas chromatography, it is then clear that surface predominated by metallic Sn does little CO2 reduction but mostly proton reduction to hydrogen gas.


Figure 1. XPS and deposition traces Figure 2. Tafel slopes and Faradaic efficiency
So oxides of Sn are responsible for CO2 reduction. Then it is synthetically feasible to generate a composite film of metallic Sn and oxides of Sn to maximize the catalytic properties. By electrodepositing Sn on Ti surface in the presence of NaHCO3/CO2, a composite film is generated and characterized by SEM (scanning electron microscopy), XRD (powder X-ray diffraction), and XPS. Crystalline SnO2 was observed in longer depositions. By monitoring of the composite thin film products, such as H2 and HCOOH, via NMR and GC of the stepped potential electrolysis, the activity of the composite film was measured and compared. The Tafel slope indicates a chemical rate limiting step with the initial charge transfer step closer to 1 equivalence of electron, but the value is not significantly different between the metallic surface and the composite surface. Current density and faradaic efficiency are both greater in the case of composite films, which shows the potentials for metal/metal-oxides composite catalysts for CO2 reduction. The author also showed that a flow cell setup can further enhance the activity of the thin film catalyst, which will be a great start to tackle the current carbon-neutral energy problem.