Title: Glycopolypeptides with a Redox-Triggered Helix-to-Coil Transition
Authors: Jessica Kramerǂ and Timothy Demingǂ§
Journal: Journal of the American Chemical Society
Affiliation: ǂDepartment of Chemistry and Chemical Biology, University of California, Los Angeles; §Department of Bioengineering, University of California, Los Angeles
Stimuli-responsive polymers are a really interesting and exciting area of research – chemists are making polymers that can respond to light, pH, temperature, oxidation, etc. These polymers have numerous applications in the biomedical field ranging from the controlled release of drugs to the amplification of signals in biosensors.
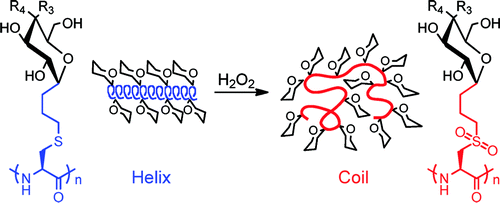
Polypeptides have an advantage in the responsive polymer field, because they can easily form ordered structures such as α-helices and β-sheets that can then be disrupted to form random coils. In general these switches in conformation are accompanied by a change in solubility. This behavior does not effectively mimic what is observed in nature, because proteins are able to undergo changes in conformation without losing solubility. In this paper, the researchers describe a new glycopolypeptide that transitions from α-helix to random coil upon oxidation, but remains water soluble throughout.
The authors first synthesized glycopolypeptides through glycosylation of L-cysteine using a thioether linkage and then polymerizing via the ring opening polymerization of an N-carboxyanhydride. The authors used (PMe3)4Co to initiate polymerization and found that they obtained polymer in excellent yields. They were also able to control chain length by controlling the amount of initiator. Their polymers also had very narrow distributions of molecular weights as determined by gel permeation chromatography. The authors synthesized both glucose and galactose modified polypeptides.
These polypeptides had excellent water solubility due to the polar glycosides. Additionally, the thioether moiety allowed for a convenient oxidation to sulfone groups, which gives the molecules a switchable characteristic. Both glucose and galatose modified polypeptides were found to be α-helical using circular dichroism (a method of determining the conformation of molecules by studying their absorption of circularly polarized light). However after oxidation both were still soluble, but had adopted random coil conformations. The authors then prepared a sulfoxide (less oxidized) form of the galactose modified polypeptide. This polypeptide did not exhibit the same change to a random coil conformation.
The researchers hypothesized that the sulfone groups are necessary to induce the change in conformation, because this change is caused by a disruption of side chain packing induced by the strong interactions between the sulfone groups and water. The authors supported this hypothesis by creating a glycosylated polypeptide that had an additional methylene unit between the thioether group and the polymer backbone. They showed that upon oxidation the polypeptide did not undergo a conformation change. This indicated that the sulfur bond in the new polymer was far enough away from the backbone that changes in interaction with water were not enough to affect the conformation.
This paper is an exciting step towards mimicking the conformational changes observed in natural proteins without loss in solubility.