DOI: 10.1021/ja308194w
Author: Iou-Sheng Ke a, Mykhaylo Myahkostupov b, Felix N. Castellano *b, and François P. Gabbaï *a
Journal: Journal of American Chemical Society
Affiliation: a) Department of Chemistry, Texas A&M University, College Station, Texas, USA; b) Department of Chemistry and Center for Photochemical Sciences, Bowling Green State University, Bowling Green, Ohio, USA.
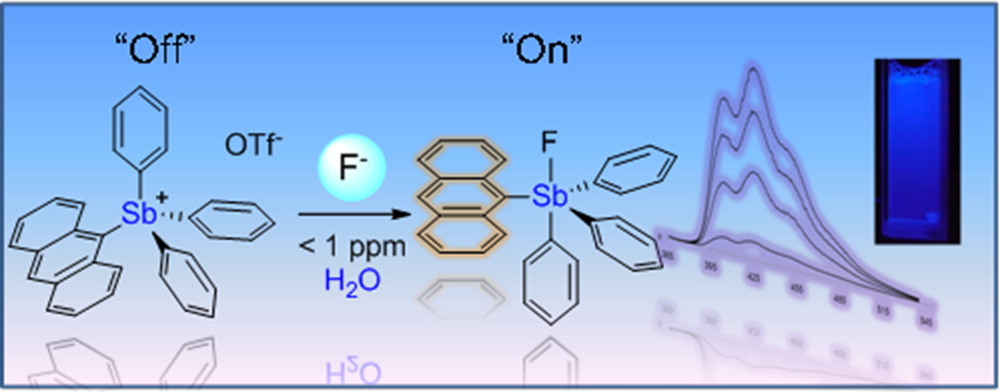
Take-home Importance According to the Authors: The 9-anthryltriphenylstibonium cation, [1]+, has been synthesized and used as a sensor for the toxic fluoride anion in water. This stibonium cation complexes fluoride ions to afford the corresponding fluorostiborane 1-F. This reaction, which occurs at fluoride concentrations in the parts per million range, is accompanied by a drastic fluorescence turn-on response. It is also highly selective and can be used in plain tap water or bottled water to test fluoridation levels.
Take-home Importance According to the Blogger: Stibonium cation is not the cation of a new element; we call the element antimony 😉 This simple complex fluoresces in the presence of fluoride and is very sensitive. Take some tweaking, but it works very well.
Tidbit from the Blogger: First, I would like to utter a word of remembrance for Neil Armstrong, who has inspired and will continue to inspire generations to bravely go forth and explore the unknown realms.
On an unrelated note, there are rather alarming reports on journal article retraction and academic integrity in Nature (10.1038/489346a) and PNAS (10.1073/pnas.1212247109). Retractions due to honest mistakes only constitute about 20% of the total number investigated, which resonated with me in particular as I just completed the Responsible and Ethical Conduct of Research (RCR), a part of my NSF fellowship requirement. In all honesty, it was one of the most informative web courses I have taken and gave me great pleasure in discovering my previous misconceptions. Strongly recommended.
At last, I recently found an old editorial on science blogs (10.1021/ac102628p) and in summary, caveat emptor. I agree wholeheartedly that all words here should be read with a grain of salt as said in disclaimers and such, since I am not yet an expert. By the way, all the editorial posts in Analytical Chemistry by Professor Royce Murray are very good. Interesting and informative read, again strongly recommended. Well now, back to chemistry.
Summary: Fluoride is an interesting ion that it is regularly added to drinking water and preventing tooth decay, but at the same time, it could be rather toxic at higher concentrations. The article itself is rather straightforward. Fluoride ions (Lewis basic) can interact strongly with trialkyl boron molecules or tetraalkyl pnictogen molecules (Lewis acidic) in some solvents while weakly in others. The weak interaction could be due to stronger ligand strength from the solvent, as predicted by the spectrochemical series. In this case, water could displace ligated flouride ions, forming a switch mechanism.
Comparing the acidity of the tetraphenyl pnictogen cations, the antimony analog is greater than the phosphorus or the arsenic analogs, due to both steric and electronic reasons. The increase in ion size allowed greater electron density polarizability and greater orbital overlapping during electron donor-acceptor interactions, as in electropositivity. In the anthryl-substituted structure, binding of the fluoride ions caused a blue shift in the UV absorption spectrum from the triflate complex. At the same time, fluorescence quantum yield was increase more than ten-fold due to decrease of nonradiative decay and removal of intramolecular ligand-metal charge transfer that might otherwise quench the fluorescence.
Well, sounds great, but the complex binds with water strongly enough to precipitate. How can this fluoride sensor function in water? The solution is simple. Just like how soap removing grease by creating bipolar micelles, a similar approach was utilized with cetyltrimethylammonium bromide as an additive. This way, the stibonium complex was kept away from water binding, but still remain soluble in aqueous environments. Remarkably, the sensor was only selective for fluoride ions and did not respond to any of the following: Cl–, Br–, I–, NO3–, N3–, HCO3–, or SO42-, avoiding a major problem of many sensors–false positive response by species with similar chemical properties. Impressively, at a concentration of at least 1 ppm, the color change is visible in about one minute.
All based on a simple organometallic complex.