Featured image and figures used with permission from Soh et al. via ACS AuthorChoice open access.
Title: Anomalous Charging Behavior of Inorganic Materials
Authors: Yan Fang, Linfeng Chen, Yajuan Sun, Wai Pong Yong, and Siowling Soh
Year: 2018
Journal: The Journal of Physical Chemistry
https://pubs.acs.org/doi/full/10.1021/acs.jpcc.8b02478
One dry early morning, the alarm clock rang you up. With half-opened eyes, you grabbed the plastic comb on your desk to quickly comb your hair. But the more you combed, the more your hair flew away from your head towards the comb. “It’s so hard to tame the flyaway hair!” You exclaimed (Figure 1).

Figure 1 The annoying flyaway hair with a plastic comb
Perhaps many of us have had the similar experience. When the plastic comb rubs against your hair, it gains a negative charge while your hair gains a positive charge. This is an example of contact electrification where materials become electrically charged after contact. Another example is rubbing a ruler with wool and hold it towards bits of papers. The bits of papers will stick to the ruler as one surface become positively charged and the other negatively charged. The two contact surfaces always have opposite charges in contact electrification. But today, researchers have found some materials that challenge this common belief – the two surfaces bear the same charges!
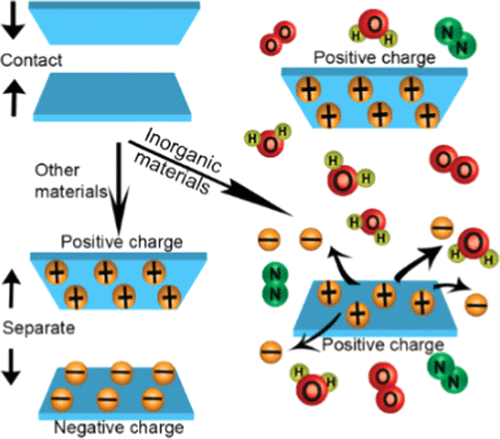
This intriguing behavior comes from inorganic materials, which are compounds without carbon. These include mica, quartz, sodium chloride, calcium fluoride (Figure 2). Researchers call this unexpected behavior as anomalous charging behavior in contrast to the common observation of contact electrification.
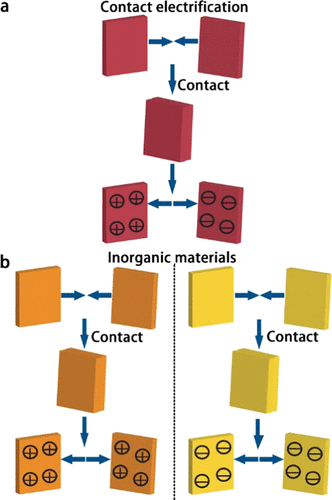
Figure 2 We normally observe the two surfaces have opposite charges after contact (a). However, inorganic materials can charge either both positively or both negatively after contact (b).
Researchers used clean and discharged materials for the experiments. They brought the materials into contact for 20 times and then measured the charges on both surfaces. They found that polymers behave as commonly expected, one surface has positive charges while the other one has negative charges. Yet, when they brought two pieces of mica together, both surfaces are positively charged. Mica is not the outlier, other inorganic compounds, such as sodium chloride (the compound in our table salt), also gain positive charges after contact with each other. Quartz, however, behaves in another way, it bears negative charges on both surfaces of contact.
Wait, if both surfaces have the same charge after contact, does that mean the materials have gained or lost charges after all? This could be a serious problem as it violates the law of conservation of charges!
This has triggered researchers into deeper investigations. By measuring how charges behave with time, they discovered the two surfaces of mica does bear opposite charges immediately (∼0.5 s) after contact. Therefore, the conservation of charges still holds for the phenomenon. But how do the negative charges on one surface flip to positive soon afterward?
They have attributed the flip of charges to the interaction with surrounding atmosphere. They discovered the percentage of oxygen (O 1s) changes before and after contact. Therefore, they suspected oxygen and/or water vapor may be responsible for the flip of charges in these inorganic materials. However, the real cause is still unknown.
Another unanswered question is why some materials tend to retain positive charges on their surfaces while some retain negative charges. For example, silicon charges positively while quartz charges negatively. They further investigated the phenomenon by contacting them with different materials. Yet, mica always has positive charges no matter it is in contact with sodium chloride or quartz. Similarly, quartz is also stubbornly negative charged always (Figure 3).
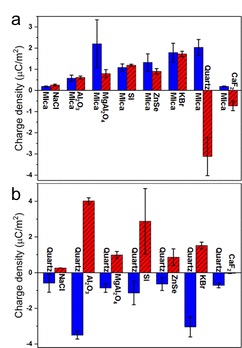
Figure 3 Charge density for contacts between (a) mica and other inorganic materials, and (b) quartz and other inorganic materials. Mica is always positively charged while quartz is always negatively charged.
This surprising observation of anomalous charging behavior has left us with many unanswered questions. Although we are experiencing contact electrification every day – from combing hair to cool tricks with ruler and paper bits, there are still many mysteries behind contact electrification. Let us look forward to further advancement and understanding of this phenomenon in future!
This is an unofficial adaptation of an article that appeared in an ACS publication. ACS has not endorsed the content of this adaptation or the context of its use.