Title: Construction of Pd-Zn dual sites to enhance the performance for ethanol electro-oxidation reaction
Authors: Yajun Qiu, Jian Zhang, Jing Jin, Jiaqiang Sun, Haolin Tang, Qingqing Chen, Zedong Zhang, Wenming Sun, Ge Meng, Qi Xu, Youqi Zhu8, Aijuan Han, Lin Gu, Dingsheng Wang & Yadong Li.
Journal: Nature Communications
Year: 2021
DOI: https://doi.org/10.1038/s41467-021-25600-9
The ethanol oxidation reaction (EOR) has the potential to be a broadly important reaction on the global path to ubiquitous renewable energy. EOR is the driving reaction behind direct ethanol fuel cells (DEFC). Fuel cells in general are beneficial because of their low carbon dioxide emissions and relatively high efficiency. Ethanol is particularly useful because it can be made renewably and it has a high energy density compared to methanol. Finding the perfect catalyst is a major challenge of the technology. Researchers at Wenzhou University in China may have found a promising catalyst by utilizing Pd-Zn dual sites (meaning both atoms act synergistically) for EOR.
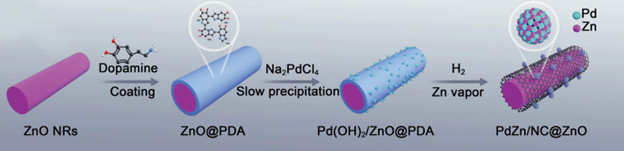
These researchers began by describing the method of fabrication of this catalyst, denoted PdZn/NC@ZnO. The ZnO represents the nanorod support. The NC is for N-doped (when an electron donating material is embedded in the carbon as an intentional impurity to modify its electronics) carbon which was created from the reduction of a polydopamine coating and is used to prevent Pd atoms from moving. Zinc vapor was created from the ZnO and was used to bind the Pd atoms thereby creating Pd-Zn nanoparticles and the dual sites. This synthesis sequence is shown in Figure 1. The authors confirmed an even distribution of these Pd and Zn in the nanoparticles by using energy dispersive X-ray spectroscopy (EDS). The average diameter of the nanoparticles was determined to be 4.75 nm through scanning transmission electron microscopy (STEM).
This catalyst (Pd-Pd dual site, PdZn/NC@ZnO) was then compared in performance to two similarly structured catalysts for EOR. One having Pd-Pd sites (Pdn/NC@ZnO) and on having individual Pd sites (Pd1/NC@ZnO). These three catalysts were also compared with commercially available Pd/C (palladium on carbon). The authors found that the novel catalyst performed better across many metrics ranging from efficiency to stability.
The authors then tested this catalyst in an actual DEFC. The PdZn/NC@ZnO performed better in terms of power density by 11.6%. The power density was even larger when normalized by the Pd loading in each catalyst. This lead the authors to believe that PdZn/NC@ZnO was an effective catalyst in real operational conditions.
Finally, the researchers investigated the mechanism that makes Pd-Zn dual sites superior to the other catalysts. They found that the adsorption of ethanol on Zn leads to a path that favors the dehydrogenation of ethanol at the O-H instead of the C-H bond. This path sets up the desorption of the final product acetate to be more favorable than on Pd-Pd sites. The five states of the EOR on both Pdn/NC@ZnO and PdZn/NC@ZnO can be seen in Figure 2.
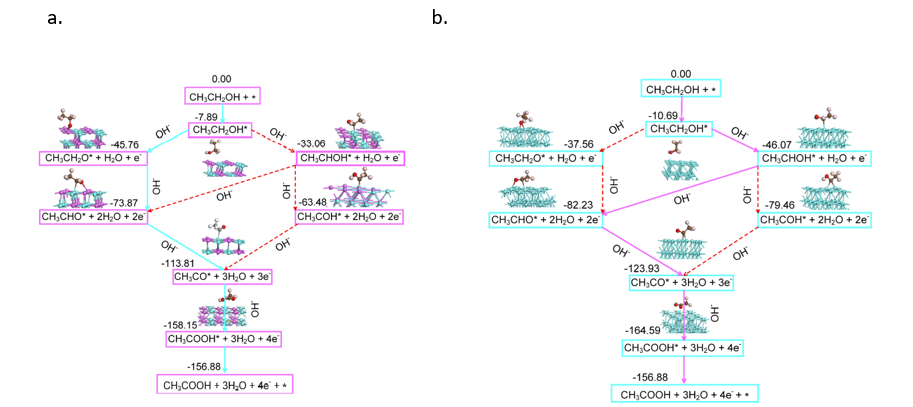
The researchers showed superior performance of a Pd-Zn dual site catalyst over Pd-Pd sites and individual Pd site catalysts for EOR. This was shown through performance tests of the catalyst and further elucidated through a mechanism investigation. While more research into alloyed nanoparticles for DEFC needs to be done to further optimize the EOR, this research offers new hope for the viability of DEFCs. These fuel cells may play a small but important role in humanity’s global fight against climate change and are worth paying attention to in the coming years.

