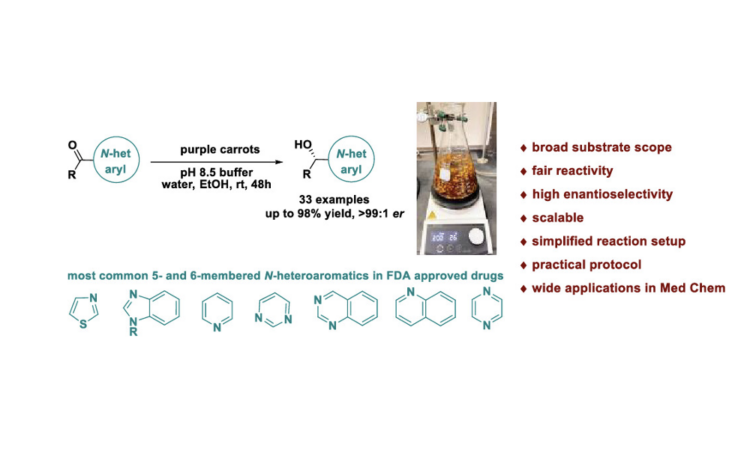
Title: Enantioselective Bioreduction of Medicinally Relevant Nitrogen-Heteroaromatic Ketones
Authors: Wen-Ju Bai, Michelle A. Estrada, Jackson A. Gartman, and Andrew S. Judd
Journal: ACS Medicinal Chemistry Letters
Year: 2023
Header image reprinted with permission from Bai, W.-J.; Estrada, M. A.; Gartman, J. A.; Judd, A. S. Enantioselective Bioreduction of Medicinally Relevant Nitrogen-Heteroaromatic Ketones. ACS Medicinal Chemistry Letters 2023, 14 (6), 846–852.
Chiral secondary alcohols are prevalent in many natural products and medicinally-relevant compounds. They also serve as valuable synthetic intermediates. Perhaps the most direct way to access these scaffolds is through the reduction of a ketone (Figure 1). A variety of these reductions exist, but they are not without shortcomings. For example, if the reduction is not enantioselective and gives a racemic product (a mixture of both the right-handed and left-handed enantiomers), you only get 50% of the product you want, and the other 50% is wasted or must be recycled. Chirality in medicinal compounds is super important, because while one enantiomer may give a therapeutic effect, the other might be toxic. Take the classic example of thalidomide; while one enantiomer has sedative effects, the other enantiomer causes severe birth defects. Some of the current methods to selectively reduce ketones to just one of these enantiomers require harsh conditions, stringent safety protocols, and expensive transition metals.
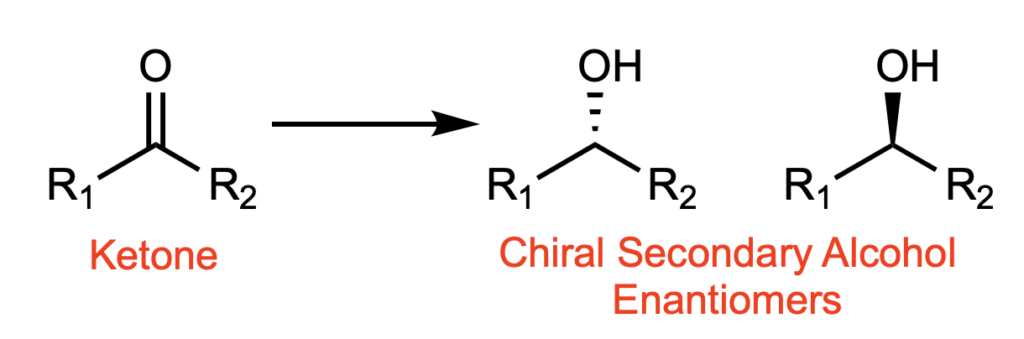
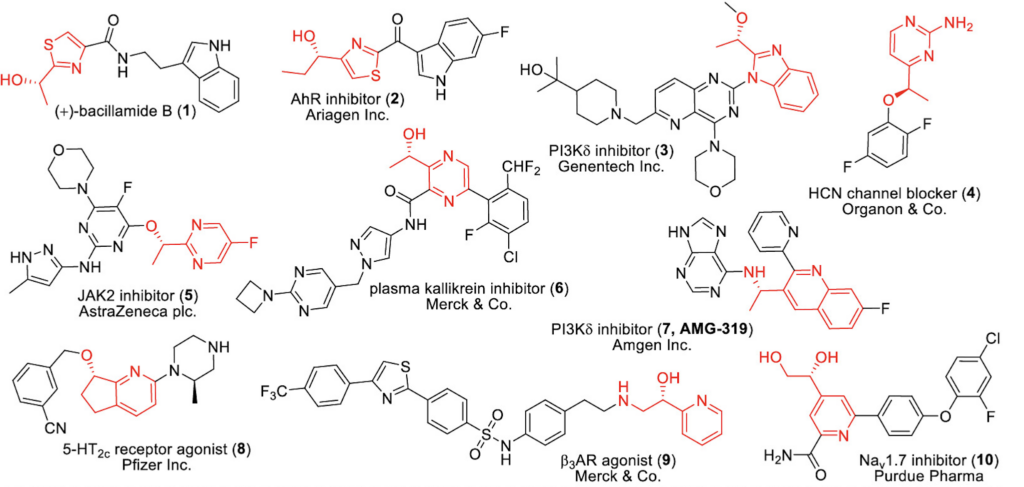
Figure 2 highlights some important medicinal compounds that contain both a chiral alcohol (or its derivative) and a nitrogen-containing heterocycle, which are both shown in red. These pharmaceutical compounds exhibit a wide array of structural complexity and biological activity. Inspired by these scaffolds and the need for a safe and cost-effective way to reduce ketones to chiral alcohols, a group of researchers at AbbVie decided to turn to nature for some help. In May of 2023, they reported that the ketone reduction can be accomplished by using purple carrots in buffered aqueous media (Bai et al.).
Plant-mediated reductions are not new, per se, but many of them suffer from low reactivity, low enantioselectivity, and a narrow substrate scope. In addition, plant-mediated reductions of nitrogen-heteroaromatic ketones have not been thoroughly studied.
Bai and coworkers screened a bunch of mild reaction conditions and found that combining the ketones with small pieces of purple carrots in a mixture of water, ethanol, and a pH 8.5 buffer solution worked the best because it gave the highest yield and selectivity. The reaction was stirred at room temperature for 48 hours. They made sure to cut the carrots into small pieces to enhance their surface area and increase the degree of ketone-carrot contact.
Overall, the reaction set-up is quick, simple, cost-effective, safe, and user-friendly. What’s more is that the reaction performs well on both a small (milligram) scale and a large (gram) scale, which is important for being able to mass-produce the alcohol products. In addition, the reaction performs astonishingly well regardless of the freshness of the carrots and the carrot manufacturer. Carrots bought from different stores including Trader Joe’s, Whole Foods, Jewel-Osco, Kroger, and Target gave similar results in terms of yield and enantioselectivity!
Using purple carrots instead of toxic chemicals sounds great, but how does this even work? It turns out that the carrots contain naturally-occurring reductase enzymes that can reduce ketones with ease. Enzymes are proteins that act as catalysts for certain chemical reactions. They also tend to favor a single enantiomeric product.
The AbbVie researchers demonstrated that this plant-mediated bioreduction can work on a variety of nitrogen-heteroaromatic compounds. The focus on nitrogen-heteroaromatics stems from the fact that many FDA-approved drugs contain these rings. Below is a just small subset of the chiral alcohols they made using this bioreduction which serve as important precursors to various pharmaceutical compounds. In general, the yields are substantial, the enantioselectivity values are excellent, and the substrate scope is broad.
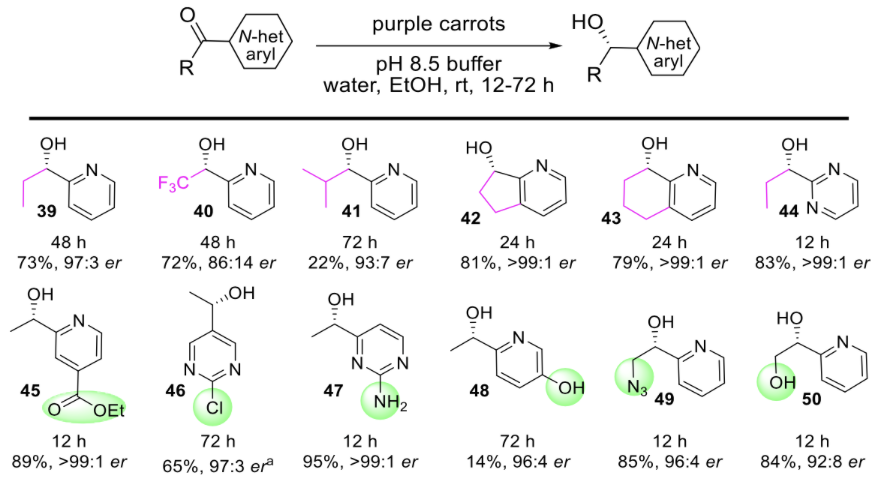
To expand the scope of the study and demonstrate the real-world application of the carrot-mediated reaction, the researchers made a variety of chiral alcohols that can serve as precursors to known pharmaceutical compounds. Figure 3 shows how alcohols 35, 46, and 49 can be converted into larger building blocks (red) for these drugs (in boxes).
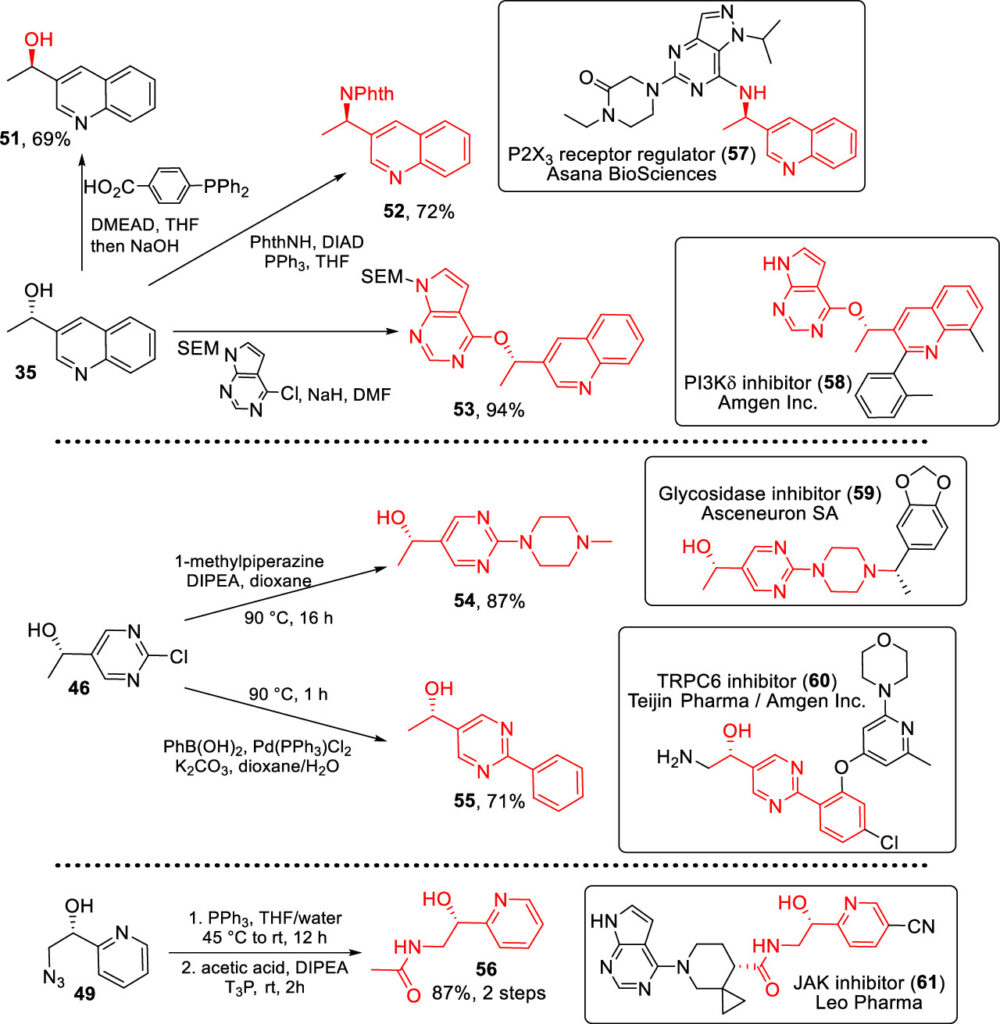
Overall, the reaction can safely and effectively convert ketones into valuable chiral alcohols by exploiting the power of nature’s catalysts found in purple carrots. Hopefully, this reaction will be implemented in complex syntheses and medicinal chemistry campaigns, sparking a paradigm shift towards greener methods of chemical synthesis.
~~~
This is an unofficial adaptation of an article that appeared in an ACS Medicinal Chemistry Letters publication. The ACS has not endorsed the content of this adaptation or the context of its use.
Figures are reprinted with permission from Bai, W.-J.; Estrada, M. A.; Gartman, J. A.; Judd, A. S. Enantioselective Bioreduction of Medicinally Relevant Nitrogen-Heteroaromatic Ketones. ACS Medicinal Chemistry Letters 2023, 14 (6), 846–852. Copyright 2023 American Chemical Society.
Full Reference: Bai, W.-J.; Estrada, M. A.; Gartman, J. A.; Judd, A. S. Enantioselective Bioreduction of Medicinally Relevant Nitrogen-Heteroaromatic Ketones. ACS Medicinal Chemistry Letters 2023, 14 (6), 846–852. DOI:10.1021/acsmedchemlett.3c00114.