Title: A Ratiometric Two‐Photon Fluorescent Probe for Tracking Lysosomal ATP: Direct In Cellulo Observation of Lysosomal Membrane Fusion Processes
Authors: Dr. Yong Woong Jun, Dr. Taejun Wang, Sekyu Hwang, Prof. Dokyoung Kim, Dr. Donghee Ma, Prof. Ki Hean Kim, Prof. Sungjee Kim, Prof. Junyang Jung, and Prof. Kyo Han Ahn
Year: 2018
Journal: Angewandte Chemie International Edition
https://pubs.acs.org/doi/10.1021/acscentsci.8b00471
When you think of a cell, often what comes to mind are the still images in a biology textbook, with every part of cell enlarged and fixed in place so that they can all be clearly labeled. But in reality, a cell is bustling with activity. Organelles (smaller structures within the cell with specific functions) are always interacting and in some cases even colliding with each other. One type of organelle that can do this is called the lysosome. The lysosome is a small, membrane-bound container for enzymes and an acidic environment that can break down waste products from the cell. It’s like the cell’s garbage disposal. It takes in unwanted items from around the cell, shreds them with the machinery and acid inside of it, and eventually dumps them outside the cell. However, lysosomes can also recycle and transport useful molecules for signaling different cells. In order to transfer cargo, a lysosome could merge into and combine with another lysosome, either permanently, in a process scientists refer to as “full-collapse,” or temporarily, in a process called “kiss-and-run.” (Hey, whoever said scientists don’t have a sense of humor?)
So far, scientists have mostly been studying the antics of lysosomes using artificial organelles, or by loading up the lysosomes with heavier fluorescent proteins or quantum dots. However, researchers at Pohang University of Science and Technology in the Republic of Korea wanted to develop a new way to see lysosomes inside of living cells, using the contents of lysosomes themselves to get a signal. They built a molecule called Lyso-ATP to do just that.
Lysosomes contain high concentrations of a molecule called adenosine triphosphate (ATP). ATP is the energy currency of the cell; it provides the molecular energy that keeps all the cells in your body running! The researchers designed Lyso-ATP to be responsive not only to ATP but also to the acidic environment of the lysosome. [The lysosome has a pH of about 5. In comparison, the rest of your body has a pH of about 7.4, which is right around neutral. The lower the pH value, the more acidic the solution is.]
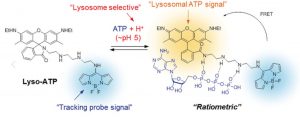
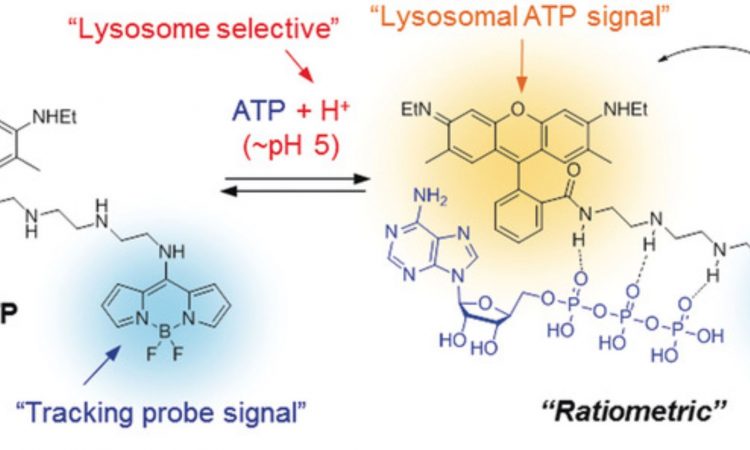
Fig. 1: The chemical structure of Lyso-ATP, both with and without ATP bound to it. When ATP is bound, yellow fluorescence is given off from the molecule. Without ATP, only the blue signal from the tracking probe portion of the molecule is observed.
Lyso-ATP has three components to it: (1) a tracking dye that continually gives off blue light, so that the scientist could know where the molecule is in the cell, (2) a linker that sticks to ATP, changing the fluorescent properties of the molecule, and (3) a lysosomal ATP signal molecule that only gives off yellow light when ATP has stuck to the linker and the molecule is in an acidic environment. (Fig. 1) Thus, Lyso-ATP should only give off a yellow light when inside the lysosome. The amount of yellow light given off compared to the blue light from the tracking probe signal could be used by scientists to calculate the pH of individual lysosomes.
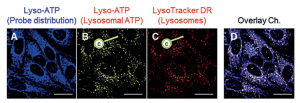
Fig. 2: Cellular localization of Lyso-ATP. The blue signal (A) shows where the probe is within the cell using the tracking probe signal. The yellow signal (B) shows only where the Lyso-ATP probe is interacting with ATP molecules inside of the lysosomes. To confirm that the yellow signal is from lysosomes, a red LysoTracker dye was used to stain the lysosomes (C). Panel (D) shows an overlay of all three colors.
Using fluorescent microscopes, the researchers were able to show that Lyso-ATP does behave how they would expect it to. Using the blue light from the tracking dye, they could see that Lyso-ATP went all over the cell. However, it only showed yellow light at the lysosomes, which show up as little pinpoints around the nuclei of the cells. This means we have a method of imaging what individual lysosomes do with a minimal effect on their natural properties and behavior.
Once they confirmed that Lyso-ATP worked, the scientists used it in two different ways. First, they zoomed way in to look at the dynamics of individual lysosomes. In particular, they were able to observe both the “kiss-and-run” and “full-collapse” processes mentioned at the beginning. The “kiss-and-run” process means that the lysosomes come and merge together briefly, exchanging contents (“kiss”) before breaking apart and becoming two separate lysosomes again (“run”). The lysosomes repeated this several times over a minute, Using Lyso-ATP, the researchers showed that using this process, the lysosomes were able to exchange their content (ATP) and evenly redistribute it between the two. Because of the way Lyso-ATP was designed, with a constant blue signal as well as a yellow signal that increases when it interacts with ATP, the amount of yellow to blue signal could be compared and used to calculate the amount of ATP present. This type of imaging is called ratiometric imaging. Using this method, the researchers were able to tell the relative amounts of ATP in each lysosome and showed that no ATP was lost during the kiss-and-run transfer process. They did the same for the “full-collapse” process, where the two lysosomes just merge together into one larger lysosome. It truly is impressive the information the researchers were able to glean about tiny particles smaller than a single bacteria, just by imaging them on a microscope.
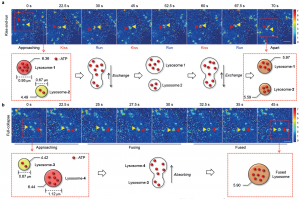
Fig. 3: Microscope images and schematics of the “kiss-and-run” process (panel a) and the “full-collapse” process (panel b).
Secondly, they were also able to zoom way out and use Lyso-ATP to visualize how lysosomes are involved in brain signaling. Besides being the energy currency of the cell, ATP is involved in neurotransmission, so visualizing it inside of the brain would give researchers a view into how it plays a role in neural pathways. Lyso-ATP is a two-photon fluorescence probe, meaning that it absorbs two photons (discrete packets of light) of low energy instead of one of high energy. It then emits some of this energy as a photon (fluorescing). The two-photon approach means that Lyso-ATP can be excited by much lower energy light compared to normal fluorescence probes. This allowed the researchers to use it to visualize thicker brain slices than have previously been studied due to the lower energy light being able to penetrate deeper into the tissue. In particular, they took a section of mouse brain around the corpus callosum, which is a band of nerve fibers that join the two halves of the brain. The images they took showed Lyso-ATP lighting up along neuronal pathways, showing that lysosomes with ATP in them were highly concentrated and active there (Fig. 4).

Fig. 4: Images of Lyso-ATP inside of mouse brain tissue sections. A slice of mouse brain was taken from the corpus callosum, stained with Lyso-ATP, and imaged using two-photon fluorescent imaging. Neuronal pathways within the corpus callosum had the highest fluorescence.
In conclusion, these researchers were able to develop a chemical tool that allowed them to monitor dynamics in living systems, both deep within single cells as well as at a larger organ level. Although the applications of this probe aren’t immediately apparent, as with a drug or a diagnostic agent, these types of chemical tools for studying basic biology are still extremely important to understand what is going on in our bodies at the most basic level. The more we understand about the body, the more we can innovate and develop new solutions for disease.