Title: Biocatalytic N-Alkylation of Amines Using Either Primary Alcohols or Carboxylic Acids via Reductive Aminase Cascades
Authors: Nicholas J. Turner, et al.
Journal: Journal of The American Chemical Society
Year: 2018
https://pubs.acs.org/doi/full/10.1021/jacs.8b11561
There is little doubt that enzymes are the most talented synthetic chemists we know. With eons of fine-tuning through evolution and selective pressure it is not surprising they can catalyze simple as well as mind-boggling transformations with remarkable efficiency and selectivity under mild, physiological conditions.
Many researchers have started appreciating the superb, inherent chemical know-how of proteins as superb catalysts for organic synthesis. This notion spawned a field, known as biocatalysis, which relies on the use of enzymes as catalysts for important, often unnatural transformations. Given an enzyme’s exquisitely tailored catalytic pocket and its mild chemical nature, this technology is admired for operating with beautiful regio- and stereoselectivities and for being compatible with greener chemistries.
Recently, biocatalysis had made its latest appearance in the area of amine alkylation. Adding alkyl groups to amines constitutes an essential step en route to many pharmaceuticals, agrochemicals, resins, and polymers, but current methods require large amounts of compounds, high temperatures, long reaction times, or the use of toxic or expensive reagents.
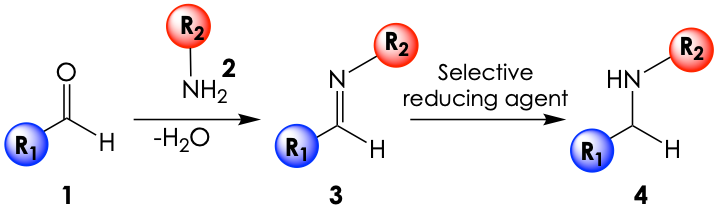
In their latest publication, the lab of Nicholas Turner at the University of Manchester has applied biocatalysis to address the issues in this arena. They draw inspiration from a common synthetic method known as reductive amination (Figure 1). Under this approach, an aldehyde (1) is reacted with a primary amine (2) to form an imine intermediate (3) which then gets selectively reduced to the wanted secondary amine product (4). While the process bypasses the use of toxic alkylating reagents directly on a primary amine, classic reductive amination is still hindered by some of the above limitations and by the fact that the parent aldehyde (1) can be unstable and needs to be prepared in situ.
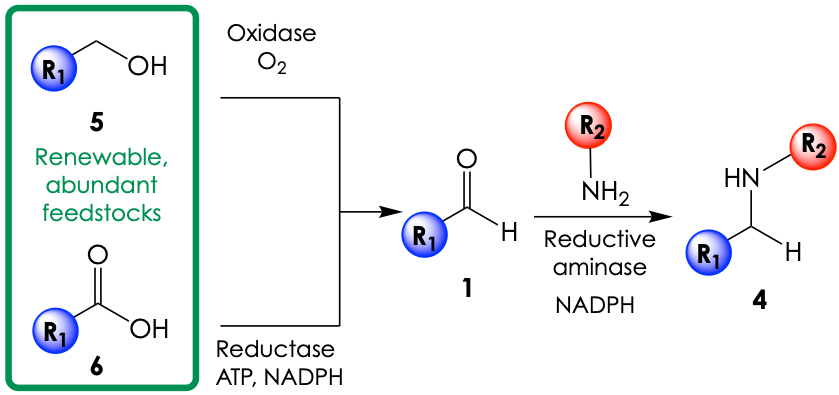
Here is where biocatalysis steps in. The team developed a reactive platform that combines three enzymes to deliver alkylated amines by two different routes. (Figure 2). The first option consists of a starting primary alcohol (5) that gets oxidized to its corresponding aldehyde (1) by an engineered oxidase enzyme called AcCO6 under aerobic conditions. The second route, in turn, opts for the reduction of yet another sustainable feedstock chemical, a carboxylic acid (6), into its aldehyde (1) with a wild-type (non-engineered) enzyme known as carboxylic acid reductase. Both reactive manifolds can meet at this point, and the aldehyde (1) can be converted to the alkylated amine product (7) directly via an enzyme-catalyzed reductive amination using a reductive aminase and the biological reductant NADPH as a source of electrons.
The rationale behind the experimental design and other experimental details are a little extensive to discuss here, but I encourage you to visit the original paper to quench that curiosity. The take-home impact this research brings is the extension of biocatalysis into a heavily exploited chemical transformation whose use is still encumbered by toxic reagents and pressing reaction conditions.
With their smart tri-partite enzymatic manifold, the authors can detour these limitations and obtain structurally diverse N-alkylated amines in excellent yields, with greener substrates, under benign conditions, and with high scalability. Also, they noticed that the product spectrum of the oxidase and the reductase overlap quite nicely with the substrate spectrum of the reductive aminase with little tendency for side reactions. This allows for an efficient single-pot reaction utilizing either the oxidase-aminase or reductase-aminase routes.
Evident limitations of this biocatalytic approach are, of course, the need for purified enzymes, which sometimes need to be engineered as well, and, in this case, the long reaction times (24 hrs). Nonetheless, it is remarkable to witness the synergy between biological systems and organic chemical platforms like reductive amination. Only time will let us appreciate the next big step in biocatalysis.