Article Title: A chemically self-charging aqueous zinc-ion battery
Authors: Yan Zhang, Fang Wan, Shuo Huang, Shuai Wang, Zhiqiang Niu & Jun Chen
Journal: Nature Communications
Year: 2020
The ability to store energy and discharge it on demand is critical for the delivery of cheap and reliable power. This is particularly important as we transition towards renewable energy sources, which are intermittent sources of energy. Integrating battery storage into the grid can help regulate power fluctuations by storing excess electricity and discharging it when energy demands exceed the amount of electricity being generated. Normally, once a battery is discharged, it needs an available power source to recharge, and that’s not always available in remote or off-grid locations. In a new paper published in Nature Communications, researchers have developed a battery that can partially recharge itself without requiring the usual source of electricity.
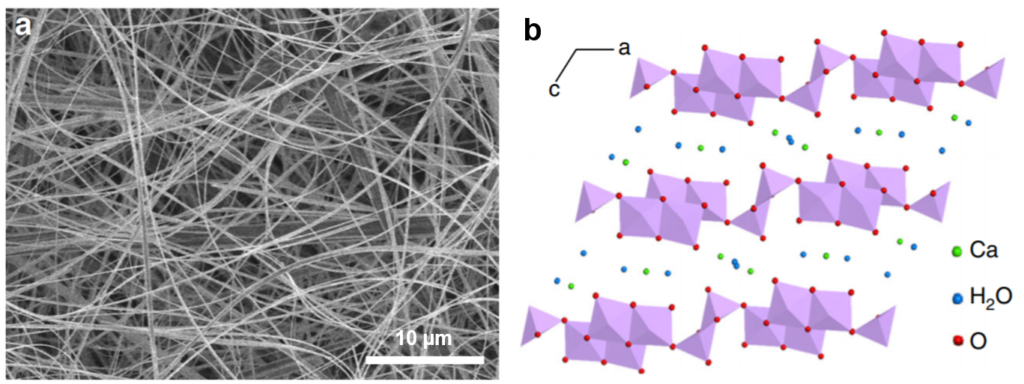
The battery created by Chen and co-workers uses a zinc nanowire electrode and a second electrode consisting of layers of vanadium oxide, shown in the figure below. The open crystal structure of this electrode, which has large channels between layers of vanadium oxide, can accommodate lots of zinc ions during the charging process, and allows zinc to move easily through large channels in the electrode during charging and discharging.
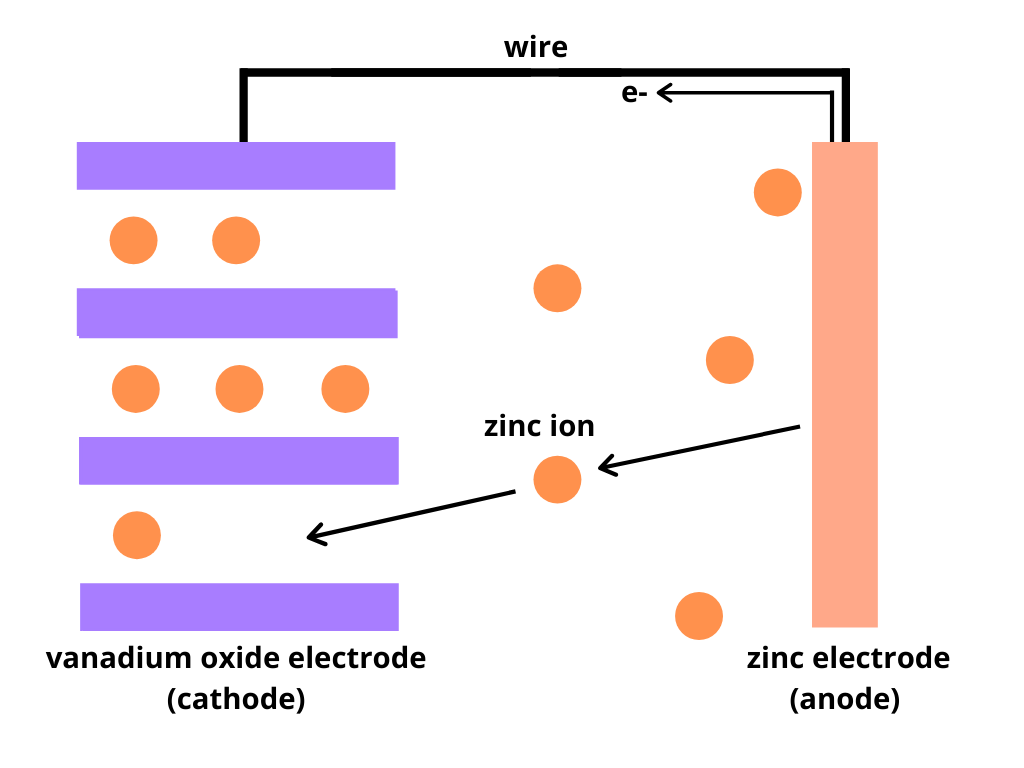
A standard battery works by using reduction and oxidation reactions to convert chemical energy into electrical energy. The discharging process in batteries readily happens at normal temperatures and pressures, meaning it is spontaneous, whereas charging is not spontaneous. In most batteries, discharging happens when cations move from the negative electrode (anode) to the positive electrode (cathode) through an electrolyte. As positive cations move across, negative electrons move through a wire in the same direction. This movement of electrons creates an electrical current. When the cations have been completely removed from the anode, the battery is completely discharged. In this battery, zinc ions need to move from the zinc electrode into the vanadium oxide electrode to produce electricity, as shown in Figure 2.
Since battery recharging is not a spontaneous reaction, energy needs to be put into the system to get the reverse reaction to happen. Running an electrical current from the cathode into the anode moves the cations from the cathode back into the anode, which recharges the battery by converting electricity into stored chemical energy. In this battery, this would mean returning the zinc ions to the zinc electrode.
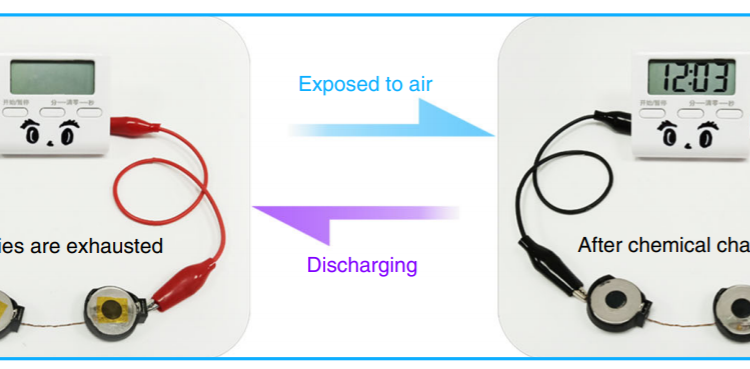
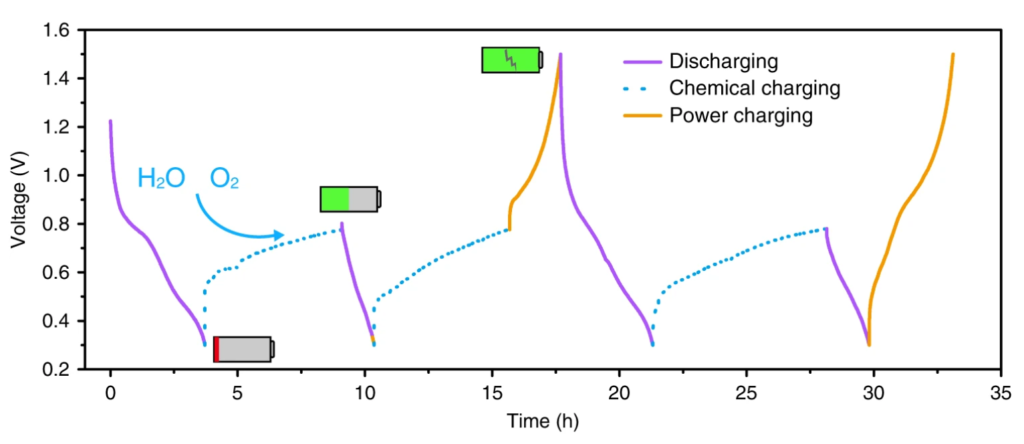
This zinc-vanadium oxide combination can work just like a normal battery and use electricity to recharge. When used like a typical battery, it showed excellent cycling stability, with no reduction in capacity after 10,000 cycles. But, electricity is not the only means of transferring energy. By using a bit of clever chemistry, a spontaneous chemical reaction can partially recharge the battery without the need for any electricity.
Here’s the gist of the chemical recharging process: vanadium needs to lose electrons, which releases zinc from the cathode. To facilitate this, oxygen and water give a little helping hand. The electrolyte in this battery contains the charged compound (CF3SO3–) in acidified water. Oxygen is dissolved into the water to provide a source of oxygen. The researchers made a coin cell battery, with a small hole in it to draw oxygen in from the atmosphere. The oxygen molecules react with the acid (H+) and each oxygen atom gains 2 electrons (e–) in the process:
O2 + 4H+ + 4e– → 2H2O
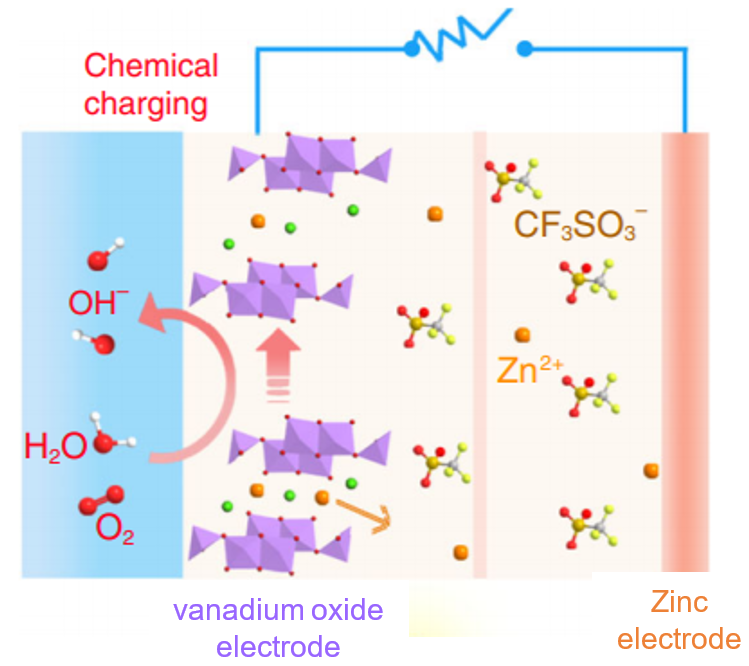
These two electrons come from the vanadium in the cathode, and this loss of electrons from vanadium triggers the release of zinc ions from the vanadium oxide electrode, shown in Figure 3. The water molecule then splits into hydroxide (OH–) and acid (H2O → OH– + H+), which replenishes the acid used to convert oxygen into water. The zinc ions and the hydroxide then react with the electrolyte compound (CF3SO3–), making a compound that attaches onto the surface of the zinc electrode. This surface attachment on the electrode is known as “plating”.
The researchers found that this self-recharging process couldn’t return the battery to full charge – after 42 hours, the battery had about 75% charge, and the charging rate had plateaued because of the plating on the zinc electrode. As shown in the in Figure 4 below, the battery can recharge itself to approximately half its capacity in about 6 hours. Luckily, this zinc-electrolyte compound can be broken down and removed from the zinc electrode by charging the battery with electricity.
Most off-grid locations require the integration of multiple devices to produce charge on demand, such as electricity from a solar panel stored in a battery system. Self-charging battery systems could circumvent the reliance on charged batteries and intermittent sources of power by providing an emergency source of power that can be generated on demand. At this stage, the technology is in its infancy, and has some issues. The chemical charging process for instance, puts a lot of stress on the battery, and loses storage capacity reasonably quickly compared to other battery systems, such as lithium-ion batteries. With more research, self-recharging batteries may become a useful alternative to standard batteries for low-energy applications and help improve energy reliability forpeople living in areas disconnected from energy infrastructure.