Authors: Linlin Jia, Lu Jin, Kai Yuan, Lingfeng Chen, Jie Yuan, Shen Xu, Wenzhen Lv, and Runfeng Chen
Journal: ACS Sustainable Chemistry and Engineering
Year: 2018
An organic light-emitting diode (OLED) is a light-emitting diode (LED) consisting of organic molecules that emit light in response to an electric current. OLED displays are light-weight, flexible, and have high brightness properties. Thus, they are widely used to make no-glare display screens, architectural surfaces, and decorative luminaires. Around 1/6th to 1/5th of the worldwide electricity produced is consumed in electric lighting. Replacing the current lighting with energy-efficient OLED could significantly reduce power consumption and eliminate several million metric tons of carbon emissions annually. There has been continuous improvement in luminous efficiencies of OLEDs. However, they have not quite reached their theoretical efficiency limits; there is still room for improvement. The present study displayed remarkable device performance and was also cost-effective, thus providing a potential OLED commercialization technique.
For a typical OLED, there are several layers, each having a different purpose:
Anode: The positive electrode through which conventional current moves into the device. It is usually made of Indium Tin Oxide (ITO), which is transparent and lets the light out of the device.
Cathode: The negative electrode from where electrons flow out. It is made up of Lithium Fluoride (LiF) layered over the Aluminium support.
Electron Transport Layer: This layer helps electron transportation from the cathode to the emissive layer. The Hole Transport Layer is similar but is between the anode and emissive layers and is used to transport holes.
Emissive Layer: This is the essential part of an OLED, where the electrons and electron holes combine to give out the emission of light.
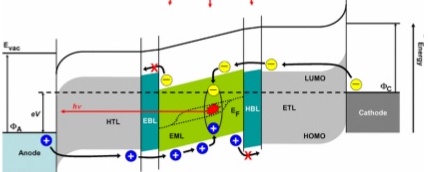
The real chemistry happens in the emissive layer. For two electrons to occupy the same orbital, they need to have opposite spins. This means that a pair of electrons in the ground electronic state will have opposite spins. These electrons occupy the ground singlet state. When molecules are exposed to radiation, these electrons can get “excited” to higher energy levels. If one of the excited electrons flips its spin in this process, it is called a triplet excited state, shown in Figure 2. When the electrons retain their opposite spins, we get a singlet excited state. Since electrons in singlet states have the same spin, they have larger electron-electron repulsions and avoid each other more. As a result, triplet excited states have less energy than the singlet excited state.
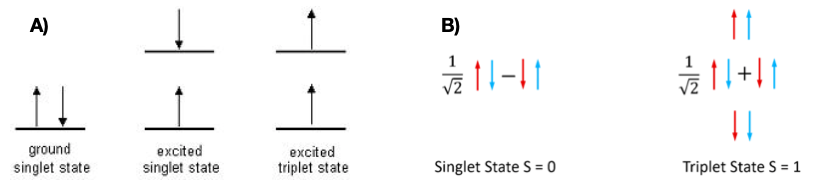
Once a molecule is in an excited state, it must return to the ground state unless it gets involved in a photochemical reaction. When the electron returns from the singlet excited state to the singlet ground state, it emits fluorescence. An electron from a triplet excited state returning to the singlet ground state emits phosphorescence. In the second case, the electron has to flip its spin orientation to come back to the ground state, meaning that phosphorescence takes a relatively longer time than fluorescence. Statistically speaking, 25% of the excited states exist as singlets, and 75% are triplets.
The organic molecules used in OLEDs undergo fast singlet emissions., but the useful emissions from the triplet states are very small. Because the triplets only emit low energy wavelengths, almost all of their energy is lost as heat. That means an OLED can have an internal efficiency of only 25% resulting from the singlets. The remaining 75% (the triplets) is just a waste. This inefficiency poses a big challenge for commercialisation..
To use that extra 75%, we can convert the singlets into triplets. Previous research has used organo-transition metal complexes such as Ir(III) complexes to convert into triplets, which also become emissive—thus reaching an efficiency of 100% through phosphorescence. However, using rare earth transition metal complexes is not favorable since they are very toxic and expensive. A new and promising technique known as Thermally Activated Delayed Fluorescence has recently been used to harvest the triplets into singlets for OLED applications. This technique harnesses 75% of the triplets with organic materials, removing the need for toxic heavy metals.
The Thermally Activated Delayed Fluorescence molecule is designed to have a small singlet-triplet energy gap, allowing for easy conversion from triplets to singlets using thermal energy. Since converting triplets to singlets takes some time, the fluorescence achieved through these triplets is slightly delayed. This is known as delayed fluorescence, but increased the efficiency of the fluorescence of the OLED to 100%.
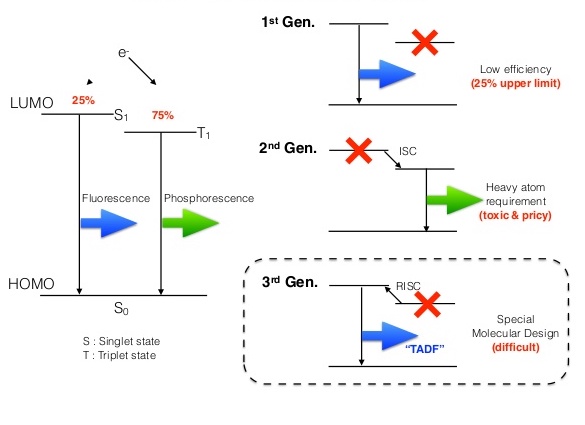
One of the severe constraints for OLED commercialization is the fabrication of the blue light emitters. Blue light, owing to its shorter wavelength, possesses very high energy. This requires a large energy gap between the ground and excited-state levels, which can eventually cause a breakdown of bonds in blue emitter molecules. This reduces the efficiency and lifetime of the device. The present study successfully achieved an ultralow turn-on voltage (a very low voltage was applied across the terminals leading to emission) for blue TADF OLEDs, although its efficiency was still low. The study obtained the most remarkable device performance for the yellow OLEDs. Moreover, since white OLEDs are fabricated using yellow and blue light-emitting molecules, this technique also gave excellent white OLED performance.
Thus, using the highly efficient TADF technique, the current study achieved materials for the fabrication of high-performance and low-cost OLEDs, which could serve as an important prototype for OLED commercialization. OLED lighting is ultra-thin, flexible, lightweight, free of glare, all along with being recyclable, free of toxic materials, and highly energy-efficient, leading to a significant reduction in energy and power consumption as well as greenhouse emissions. Thus OLEDs are the future of lightning. Improving their performance efficiency while decreasing their costs is an active area of research.

