Title: Precisely Structured Nitric-Oxide-Releasing Copolymer Brush Defeats Broad-Spectrum Catheter-Associated Biofilm Infections In Vivo
Authors: Zheng Hou, Yang Wu, Chen Xu, Sheethal Reghu, Zifang Shang, Jingjie Chen, Dicky Pranantyo, Kalisvar Marimuth, Partha Pratim De, Oon Tek Ng, Kevin Pethe, En-Tang Kang, Peng Li, and Mary B. Chan-Park
Journal: ACS Central Science
Year: 2020
https://pubs.acs.org/doi/10.1021/acscentsci.0c00755
Much of modern medicine relies on invasive procedures such as inserting an IV, a vaccine injection needle, or a ventilator. It is not uncommon for such a procedure to result in a bacterial infection, which can slow or reverse patient recovery process and may even result in death. Normally the barrier of the skin protects the body from bacteria that cause infections, but when a foreign object pierces the skin that is no longer true. The issue of device associated infections is exacerbated by the fact that the microflora of the body contain many types of bacteria, and that Gram-negative bacteria in particular are resistant to many antibiotics. As such, biofilms (groups of bacteria that stick to each other and cause infections) are a serious issue in the medical device industry. A common solution to deal with biofilms is applying a coating to the medical device, such as silver. Last year Huo et al. designed a new biofilm coating to help combat the ever-present issue of bacterial infection caused by medical devices.
Catheters are particularly useful for testing new anti-biofilm coatings, as they are easy to implant in test models, and are one of the most commonly implanted medical devices. An ideal coating, as defined by these authors, should be broad-spectrum antifouling (meaning protein and other debris from the bloodstream won’t adhere to it and make it less effective), broad-spectrum antibacterial, nontoxic, nonthrombogenic (meaning it doesn’t cause blood clots), and lasts as long as the device does. Understandably, considering all these requirements, no such ideal coating has yet been developed. Several types of catheter coatings have been trialed, including hydrophilic ones, silver ones, and antibiotic-releasing ones; however, none have met all the previously described criteria.
The approach that Hou et al. took while designing a new anti-biofilm coating is centered around the idea that a coating could be engineered to release a gas that kills bacteria, which would prevent biofilms from forming. The gas that they chose to use for their new coating is nitric oxide (NO). Nitric oxide is a colorless gas composed of one nitrogen atom double-bonded to one oxygen atom with an unpaired electron, or free radical, on the nitrogen. Nitric oxide is a good choice as it is antibacterial and endogenous to the body (is a naturally produced chemical in the body and thus is biocompatible). Additionally, if NO is released from a coating in the absence of bacteria, it will react in less than a second with the molecular oxygen in the surrounding blood, minimizing potential side effects. However, previously-developed coatings that release NO were toxic because the NO-releasing material itself was toxic, and/or prone to fouling as they were hydrophobic, because proteins would accumulate in that hydrophobic environment. As such, Hou et al. suggested that an ideal coating could be made by putting a layer of antifouling material over a layer of NO-releasing material, which would allow the NO to bubble up from within, preventing the bloodstream from interacting at all with the NO-releasing material, neatly dodging both previous issues.
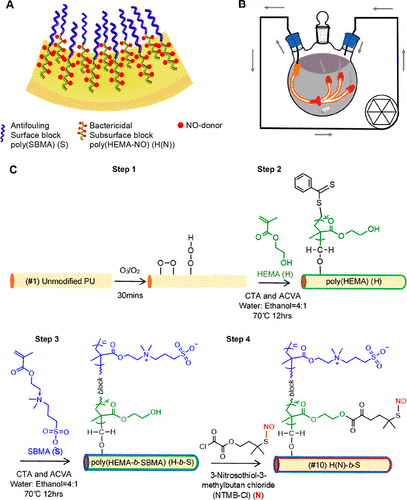
The coating that Hou et al. created for their test catheter is a diblock copolymer, meaning that the two long-chain layers of the coating are bonded to each other on a molecular level (Figure 1, A). Using flow chemistry, or circulating the reacting solution continuously (Figure 1, B), they prepared the catheter surface with ozone gas. Next, they polymerized a layer of a material called poly(HEMA) that would release the NO from the catheter surface.
After that, they repeated the ozone-initiated polymerization again on top of the poly(HEMA) to add poly(SBMA). Poly(SBMA) is an antifouling material that forms what they termed as a brush, as the long chains of the poly(SBMA) would lay flat and drape themselves over each other, protecting the poly(HEMA) from protein and bacteria that would otherwise clog it up (Figure 1, C). Because NO is a gas, it will bubble out of the brush freely, and the two parts of the diblock polymer are able to work together to both release NO gas and be non-toxic and anti-fouling. Finally, they used a material that releases NO gas called RSNO to prime the poly(HEMA) so that it had NO to release.
Once Hou et al. had synthesized their biofilm, how did they know if it had actually worked? They used a technique called attenuated total reflection Fourier-transform infrared spectroscopy to confirm that the double-layer reaction process had succeeded, and used scanning electron microscopy and atomic force microscopy to check whether their polymer entirely covered the catheter. Then they used several more specialized tests to confirm that the outer poly(SBMA) was hydrophilic, as intended, that there was no leaching of the poly(HEMA) out of the coating, that the coating was stable in water or biological serum, and that the NO is released from the poly(HEMA) layer for an extended period (15 days).
Upon testing their new catheter coating, Hou et al. found that it prevented biofilm formation by more than 99.9% over the course of thirty days by a variety of Gram-positive and Gram-negative bacteria, including several antibiotic-resistant strains. During protein anti-fouling tests, their coating exhibited very low fouling as compared with other coatings that allowed for the release of NO, which demonstrated that their two-layer compound was successfully preventing fouling as intended. Finally, in mouse and pig models their catheter showed no long-term systemic toxicity, and no evidence of thrombic or biofilm activity. Importantly, the amount of bacteria on their test catheters was reduced by more than 99.99% compared to a control catheter.
Hou et al.’s novel coating has proven extremely effective in model systems and able to meet every requirement of their definition of an ideal medical implant coating. Hopefully in the coming years we will see it, or others inspired by it, used to reduce device associated infections and improve patient outcomes in clinical settings.
Cover image by user “pat” from freeimages.com

