Title: Sila-Ibuprofen
Authors: Florian Kleemiss, Aileen Justies, Daniel Duvinage, Patrick Watermann, Eric Ehrke, Kunihisa Sugimoto, Malte Fugel, Lorraine A. Malaspina, Anneke Dittmer, Torsten Kleemiss, Pim Puylaert, Nelly R. King, Anne Staubitz, Thomas M. Tzschentke, Ralf Dringen, Simon Grabowsky, and Jens Beckmann
Journal: Journal of Medicinal Chemistry
Year: 2020
DOI: https://dx.doi.org/10.1021/acs.jmedchem.0c00813
Everyone is familiar with pain killers, especially ibuprofen. It was invented in the 1960s as an analog of aspirin. By 1985, more than 100 million people in 120 countries had received it for pain, fever, headache, arthritis etc. Pain in the body is caused by the synthesis of prostaglandins from arachnoids, brought about by COX-I and COX-II enzymes. COX-I is always present and its inhibition damages mucous layers and results in ulcer formation and other gastrointestinal issues. However, COX-II is active only during inflammation and its inhibition reduces the pain without the side effects. Ibuprofen’s ability to selectively inhibits COX-II enzyme contributed to its popularity.
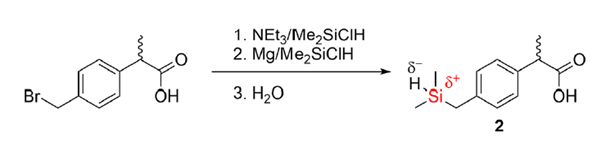
Ibuprofen has a low solubility due to its high melting point and high melting enthalpy. This greatly limits its use as a non-steroidal anti-inflammatory medicine as it cannot be administered intravenously for postsurgical pain. The scientists Kleemis et al., however, have modified ibuprofen by employing a carbon-silicon switch. Using 2-[(4-bromomethyl) phenyl] propionic acid and dimethylchlorosilane (Me2SiClH) in combination with triethylamine (NEt3), they first inserted a silyl ester to protect the carboxylic group followed by the reaction of bromoethyl group in presence of magnesium and Me2SiClH to insert the silicon atom at the desired location. Subsequent treatment in an aqueous medium deprotected the carboxylic group to give the final product, sila-ibuprofen (shown in Scheme 1).
The melting point and melting enthalpy of sila-ibuprofen was found to be 45-47°C and 15.8 kJ/mol compared to 74-77°C and 26.7 kJ/mol of ibuprofen. Consequently, the solubility of sila-ibuprofen is 83 mg/L compared to 21 mg/L of ibuprofen. IC50 value (or potency) is the concentration required to bring about a 50% inhibition in the enzymatic activity. In the case of ibuprofen, it translates to the concentration of ibuprofen at which 50% of the COX-I or COX-II enzymatic activity is inhibited. IC50 values of sila-ibuprofen suggest that it retains the same potency as ibuprofen. . The lower this value, the better, as we can then administer a lower dose. Kleemiss et al. found that like ibuprofen, sila-ibuprofen also inhibits COX-II more selectively than COX-I. The IC50 of sila-ibuprofen and ibuprofen is 8.3 µM and 3.3 µM for COX-II while it is 26 µM and 34 µM for COX-I respectively.
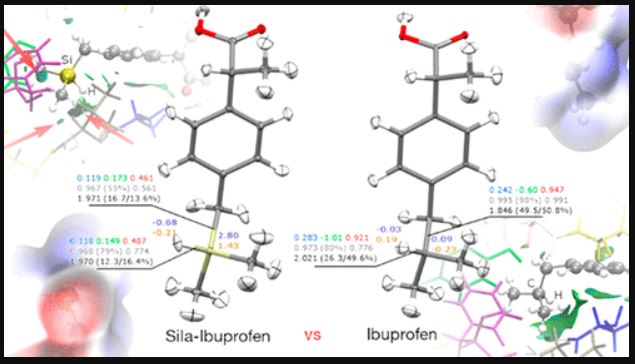
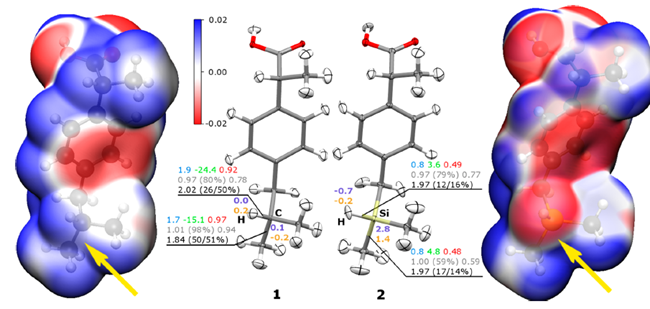
Sila-ibuprofen and ibuprofen have similar molecular and solid-state structures, based on x-ray diffraction experiments and there is a 7% change in molecular volume based on the molecular isosurfaces. The H-C and H-Si bond lengths differ by 0.374 Å and the bond angle by 0.68°. Sila-ibuprofen shows a negative electrostatic potential (the potential of a proton at that location in the molecule) around the silane hydrogen atom whereas the same area has a positive potential in ibuprofen. Furthermore, sila-ibuprofen has a greater internal charge separation. This change in the electron density around the C/Si atoms (shown in Fig.1) and bond properties reflects in the change in physical properties.
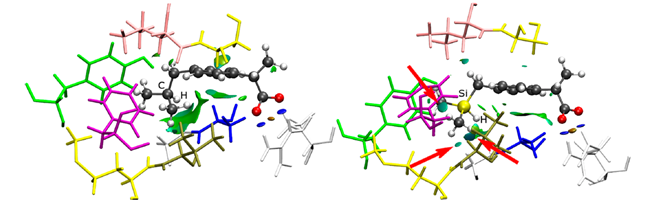
Molecular dynamic simulations revealed that for the two compounds, the interaction of carboxylic acid group with the arginine residue on COX-II is key to its recognition. The interactions of the phenyl rings with glycine-alanine residue and those involving the C-H and Si-H bonds make ibuprofen more stable than sila-ibuprofen, but the stability of sila-ibuprofen is due to the interactions of methyl and methylene groups next to silicon with methionine-valine, phenylalanine and seine-leucine residues on COX-II (shown by arrows in Fig.2). The small energy differences in binding of ibuprofen and sila-ibuprofen to COX-I and COX-II compared to the total binding energies explains the similar IC50 values of the molecules. Besides this, the small destabilization of sila-ibuprofen in COX-I compared to the small stabilization in COX-II makes it a promising candidate for drug development.
For a drug to be accepted as a medicine, one must also keep in mind its potential toxicity. Studies on cell lines showed that sila-ibuprofen has a low toxicity, that at concentrations of 300 µM, it does not affect cell proliferation or cell viability. There was also no change in the cell morphology or any extracellular activity at concentrations of 1000 µM. The lack of differences in the toxicity parameters observed for ibuprofen or sila-ibuprofen suggest that there is no toxicity to be expected when sila-ibuprofen is used in vivo. Further in vivo studies are required to understand the pharmacological effects of sila-ibuprofen.
The carbon-silicon switch is an example of a bioisosteric replacement. A bioisosteric replacement is one where one atom or a functional group is replaced by another with similar physical or chemical properties to bring about similar biological effects. This can result in enhanced physical or biological properties as in this case, where the silicon substitution enhanced its physical properties.
Reprinted with permission from Kleemiss, Florian, et al. “Sila-ibuprofen.” Journal of medicinal chemistry 63.21 (2020): 12614-12622.. Copyright 2020 American Chemical Society.