Title: Chemo-Biological Upcycling of Poly(ethylene terephthalate) to Multifunctional Coating Materials[ME1]
Authors: Hee Taek Kim, Mi Hee Ryu,Ye Jean Jung, Sooyoung Lim, Hye Min Song, Jeyoung Park, Sung Yeon Hwang, Hoe-Suk Lee, Young Joo Yeon,
Bong Hyun Sung,Uwe T. Bornscheuer, Si Jae Park, Jeong Chan Joo, and Dongyeop X. Oh
Journal: ChemSusChem
Year: 2021
https://doi.org/10.1002/cssc.202100909
Featured image from Pixabay free-use image collection.
Plastics are ubiquitous in packaging, consumer products, and industrial processes. Since they were designed to stand up to a wide range of conditions, plastics are often hard to break down once they are thrown away. Because of this, relatively little of the plastics we use are ultimately recycled because of the processes used for recycling are often not economical.
Recently, researchers from South Korea published a method to degrade Polyethylene terephthalate (PET) and reform it into potentially valuable anti-microbial coatings. Polyethylene terephthalate, shown in Figure 1, is a polymer connected by ester groups. These ester groups have traditionally been cleaved by chemical processes, but the process requires high temperature and pressure and tends to result in a mixture of different side products including Bis(2-Hydroxyethyl) terephthalate (BHET), 2-Hydroxyethyl terephthalic acid (MHET), and terephthalic acid (TPA) (Figure 1). TPA can be chemically modified to form valuable chemicals, but the complex mix of products necessitates complicated and expensive purification steps.
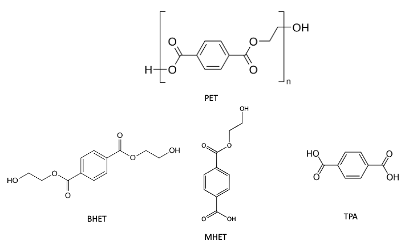
Figure 1: The polymer PET can be broken down into monomers BHET, MHET, and TPA, shown here, by cleavage of the ester bonds. Images used under creative commons license thanks to Wikimedia users Schippmeister (PET), Dschanz (BHET), Smokefoot (MHET), and Jynto (TPA)
As an alternative to chemical decomposition, a variety of researchers have suggested using microbes with enzymes capable of breaking down the PET polymer. While this process requires much gentler conditions that chemical degradation it often still results in a mixture of byproducts. In this paper, the researchers propose the chemical degradation of PET to BHET using an environment that is suitable for the later addition of bacteria. The bacteria can then use its proteins to convert the BHET to TPA and later TPA into catechol (Figure 2), a chemical that can be used to help form coatings of organic and inorganic materials.
Figure 2: Catechol can be formed from TPA using genetically modified E. coli . Catechol is useful in a variety of coatings.
The researchers used a enzyme called Bacillus subtilis esterase (Bs2Est) to convert the BHET to TPA. They tested this enzyme in both a crude mixture of the byproducts of PET chemical decomposition, which included a mix of BHET, MHET, TPA, and short chains of repeating BHET units (oligomers), a partially purified mix of only BHET, MHET, and TPA, and a fully purified mix of BHET only. The researchers measured the evolution of BHET to TPA using infrared and carbon NMR spectroscopy (Figure 3). It was found that a slight amount of excess TPA was formed in the mixtures because the enzyme was able to degrade some of the non-BHET products in the mixture .
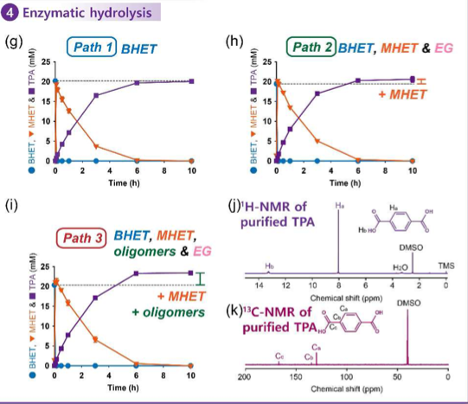
Figure 3: When exposed to Bacillus subtilis esterase, BHET is first converted to MHET and then TPA. Solutions with a mix of starting material produced slightly more than 100% yield (represented by the dotted line) because the enzyme was able to degrade oligomers to some degree.
To transform the TPA to catechol, the researchers added some of the TPA they had produced to a solution of E. coli bacteria that had been genetically modified to produce catechol. Catechol can form a thin film on a variety of surfaces, and in this study the researchers used it to form thin films on aluminum, PET plastic, and Teflon before removing the E. coli. Infrared spectroscopy and the contact angle between the coated surface and a drop of water were used to determine that each surface was successfully coated. Catechol can be used to create a linking layer between the base material and different antibacterial solutions, so solutions of chitosan, silver nitrate, or polylysine, all compounds known to have antibacterial properties, were then coated on the chitosan. With the exception of the polylysine film, these films were shown to have antimicrobial properties when placed in a fresh E. coli solution.
This paper demonstrated a pathway for turning abundant plastic waste from PET fiber into a useful and potentially valuable product for use in coatings. While the goal of this research was not to create a commercializable product, the authors urge further work into using the enzymes and bacteria demonstrated in this method to find further ways of providing value to recycled plastics. As plastic pollution becomes a growing environmental issue, biological degradation pathways could provide solutions.

