Authors: Y. Jiao, Y. Qiu, L. Zhang, W.G. Liu, H. Mao, H. Chen, Y. Feng, K. Cai, D. Shen, B. Song, X.Y. Chen, X. Li, X. Zhao, R. M. Young, C. L. Stern, M. R. Wasielewski, R. D. Astumian, W. A. Goddard III & J. F. Stoddart
Journal: Nature
Year: 2022
Featured image from Bielawski and Chen.
Every time you open a door with a key, you are experiencing a recognition event. The lock recognizes the grooves of the key and allows a turn to happen and the door to open. However, this is only allowed because the key shape matches exactly that of the lock pattern. If the key had a slightly different shape, the door would not recognize it and would remain closed. Such recognition interactions not only happen at the macro-scale, but can also occur between molecules through their interactions with each other, often termed molecular recognition.
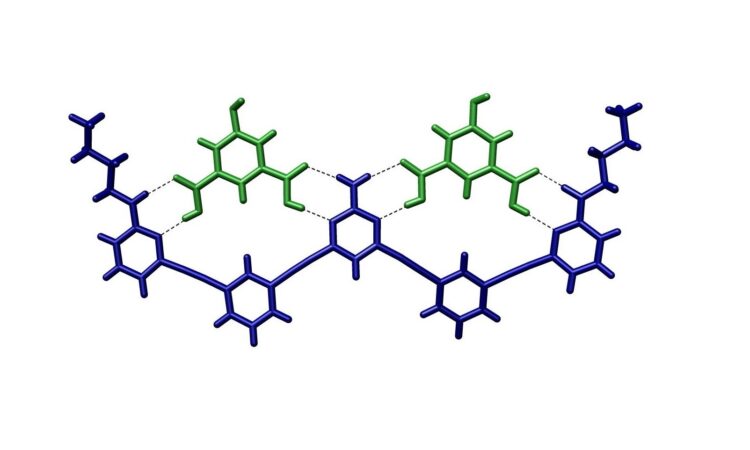
Supramolecular chemistry is the field that studies how molecules assemble and interact with each other. Molecular recognition is one of the branches that supramolecular chemistry covers. Molecular recognition is commonplace in biological systems, particularly inside the human body (Figure 1). Molecular recognition is how receptors in our brain can identify molecules such as serotonin and endorphins, which make us feel happy and euphoric. It is also how viruses are detected, through the interaction of our antibodies with viral antigen molecules. Therefore, molecular recognition is a crucial biological process that maintains the proper functioning of our bodies.
Researchers have been trying to mimic these processes using engineered molecules to facilitate technological advances in medicine and material engineering. However, it was not until recently that a group of scientists was able to successfully design a switchable molecular recognition system through the use of electricity.
Jiao and co-workers synthesized a pair of charged molecules containing bipyridine (BIPY) groups that under ambient conditions would not interact with each other because of repulsive forces (Figure 2), just like a faulty key would not interact with a door lock.
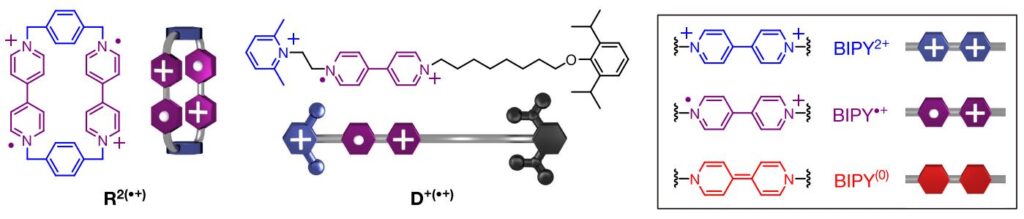
Nonetheless, this unfavourable interaction can be overturned if an electron is injected into one of the complexes via reducing agent or electricity. This decreases the energy barrier for molecular interaction (turning a cationic BIPY unit into a neutral state, purple to red, shown in Figure 2) and allows these two molecules to recognize themselves and form a supramolecular complex stabilized by radical-radical interactions (Trisradical complex, Figure 3) just like a key fitting in a lock. Furthermore, this complex could donate and recycle the extra electron used to start the recognition event to another un-complexed molecule (steps 4-to-2, Figure 3). This triggered a chain reaction that facilitated the molecular recognition of the ensemble, demonstrating that the process could be initiated with very little current.
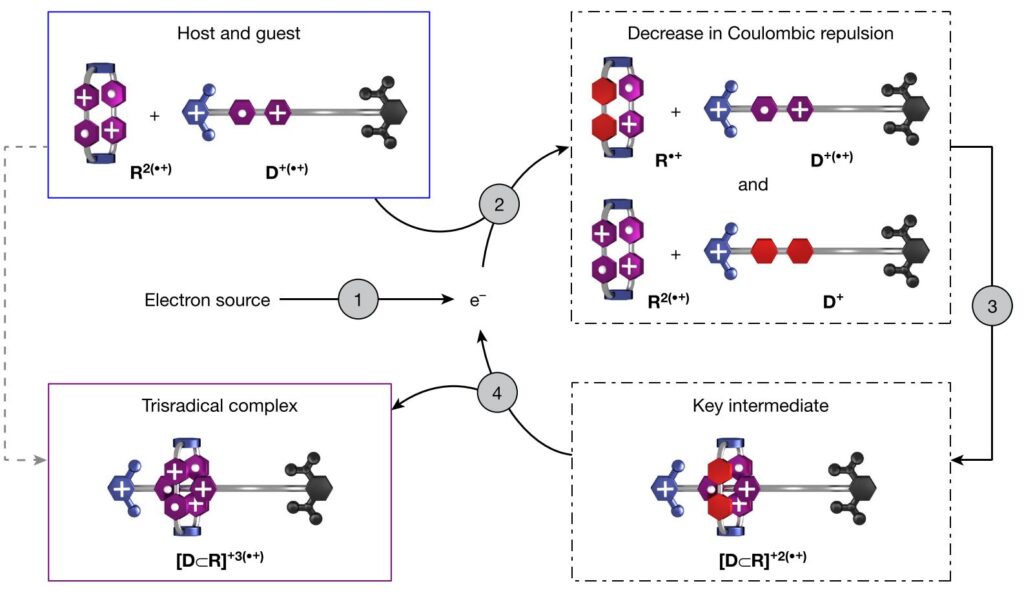
Advantages to using electricity to catalyze reactions are that electrons do not produce any waste and reaction rates can be controlled by simply adjusting the voltage. In fact, even the ratio of complexed vs. free molecules could be controlled by the amount of current supplied to the medium.
Overall, molecular recognition is an important effect that can bring many benefits in the sensing, imaging, and drug delivery field. Jiao et al. are starting to provide fundamental insight into what lies at the core of molecular recognition events and how these supramolecular interactions can be fine-tuned. This fine reaction control coupled with a renewable source of electrons can have a major impact on sustainable molecular recognition technologies.