Title: From Textile Coloring to Light-emitting Electrochemical Devices: Upcycling of the Isoviolanthrone Vat Dye
Authors: Tatiana Ghanem, Kwang Keat Leong, Hwandong Jang, Alexis Hardouin, Dr. Philippe Blanchard, Prof. Dominik Lungerich, Dr. Pierre Josse, Prof. Eunkyoung Kim, and Dr. Clément Cabanetos
Journal: Chemistry, An Asian Journal
Featured Image: Textile and Fibre Technology, CSIRO
Textile dyes are omnipresent in our clothing and household goods. While modern organic dyes allow us a wide range of color choices for textiles, they also have a downside. 200,000 tons per year end up entering the environment in untreated wastewater, leaving ecosystems vulnerable to negative effects. Recently, a team of researchers from France and South Korea developed a method to upcycle to purple dye Vat Violet 10 in waste water into a light-emitting electrochemical cell.

Figure 1: Vat Violet 10, like the more familiar indigo dye, is a vat dye. It can be reduced in a vat or other container to become soluble in water before the fabric is submerged and dyed. The color is then made permanent by reoxidizing the dye.
To accomplish this, the researchers first took Vat Violet 10 and reduced it to the form used to dye clothing. After clothing is exposed to the dye in this state, the excess is typically thrown away. The researchers, instead, added an organic solvent and a side chain to form a new molecule, 9,18-dialkoxylated 1.2,8.9-dibenzoperopyrene (DBP).
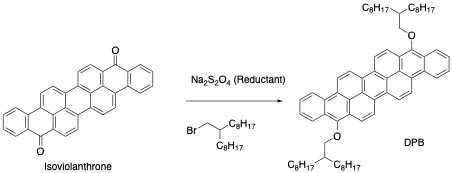
Figure 2: Vat Violet 10, also called isoviolanthrone, was reduced and then alkylated to increase its favorable properties for light emitting devices.
DBP has slightly more electron density in the molecular core than the parent compound and is able to absorb visible light as a result. The molecule can also photoluminescent, giving off a green-yellow light. DBP was put into a light-emitting device. The device was able to turn on and produce light, but the amount of light emitted was low. The researchers attribute this to DBP molecules getting stuck close to each other because the aromatic rings “stack” on each other. By decreasing the amount of active material in the device from 18% to 2%, the amount of light given off was improved.

