Title: Carbonate-Induced Electrosynthesis of Hydrogen Peroxide via Two-Electron Water Oxidation
Authors: Sotirios Mavrikis, Maximilian Göltz, Stefan Rosiwal, Ling Wang, Carlos Ponce de León.
Year: 2022
Journal: ChemSusChem
Cover image credit: Rafael Classen
Hydrogen peroxide (H2O2) is an important commodity chemical with a broad scope of applications, including bleaching, water treatment and chemical synthesis. H2O2 is produced by the anthraquinone process, which is energy intensive and produces highly concentrated H2O2, which poses safety concerns in terms of stability, storage and transport. This is wasteful, since very few applications require concentrated H2O2. There is understandably a growing interest in new technologies capable of greener H2O2 production. Researchers are taking a number of approaches to try and achieve this, including greener synthesis routes and new reactor designs that are more energy efficient, providing a sizeable step towards making this exciting new technology viable.
H2O2 is a viable candidate for this as it can produced in relatively good quantities using environmentally friendly methods. There are a couple of promising options, with arguably the most promising green route being the electrochemical oxidation of water. Here, water in an electrochemical cell undergoes a two-electron oxidation, which produces H2O2 at the electrode surface. This has a number of intrinsic benefits of existing methods, as water is the only starting material and the only oxidant is electrical energy. If this can be combined with energy from renewable sources, the process is highly environmentally friendly thanks to a low energy input and no need for harsh chemical reactants.
An interesting opportunity in future Chemical Engineering solutions is in the decentralised production of strategic materials, i.e. making the precise amount of material need at the site of use so it can be immediately used rather than having to be bought, diluted or processed. In this way, carbon, energy, and financial costs associated with storing and transporting the chemical are removed. Many of the synthesis methods used for this concept are also far greener than current environmental methods, making the whole process far more cost effective and environmentally friendly. This technique has been proposed for a number of useful materials for industrial processes or chemical synthesis. They can also provide energy security to intermittent renewable energy sources – during sunny days excess solar energy produces a biofuel, then at night the biofuel is used to supplement the energy grid.
H2O2 is a promising candidate for decentralised production, since most users only want a relatively low concentration that it well within the theoretical scope of green electrochemical synthesis. Unfortunately, this reaction is in competition with the facile four-electron oxidation of water to oxygen gas. This is particularly problematic at high reaction rates, making the process energetically wasteful under practically relevant conditions. Dr Mavrikis and co-workers have addressed this challenge by producing H2O2 via a more stable intermediate, peroxymonocarbonate. This helps to navigate around the wasteful oxidation of water to oxygen gas, making the process less wasteful. The achieved this by combining an already high performing boron-doped diamond catalyst with concentrated carbonate electrolyte, which favours the production of peroxymonocarbonate by the mechanism shown in Figure 2.
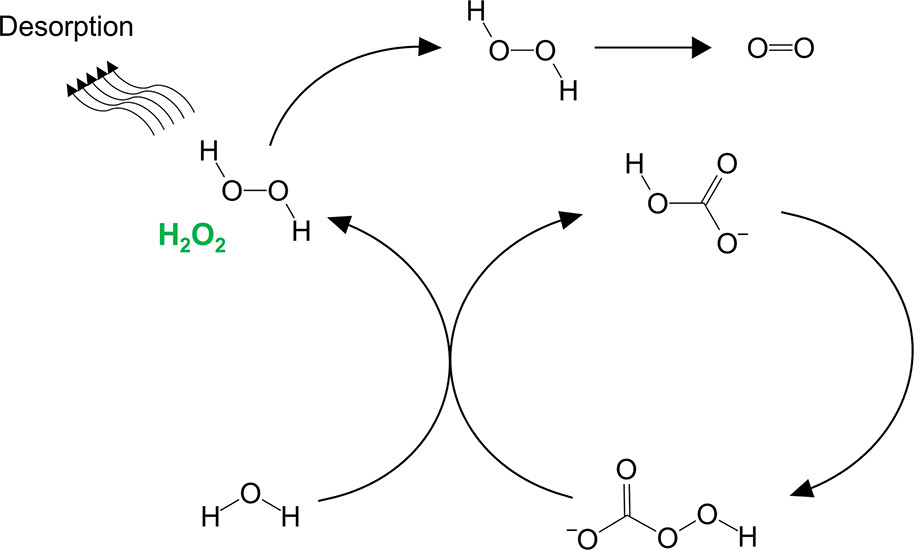
Figure 2. The authors propose a carbonate-mediated mechanism for H2O2 production that enhanced selectivity at high carbonate concentrations. Bicarbonate (HCO3–) is oxidised to peroxymonocarboante (HCO4–), which then reacts with water to liberate H2O2 Image reproduced with permission from Mavrikis et al. copyright American Chemical Society, 2021.
They were able to demonstrate, by simply increasing the concentration of carbonate, they could record a 26.5% increase in H2O2 selectivity up to an impressive 91.5% even at high reaction rates. The authors state this represents a new carbonate-mediated reaction path to H2O2 that requires less energy versus the typical water oxidation route. This concept could allow users to produce working solutions on site as and when they are needed.
Interestingly, H2O2 can also be made by the electrochemical reduction of oxygen gas. This presents the possibility of simultaneously using the technology developed here for water oxidation alongside other catalysts designed for water reduction to give twice the production of H2O2 from the same reactor design.
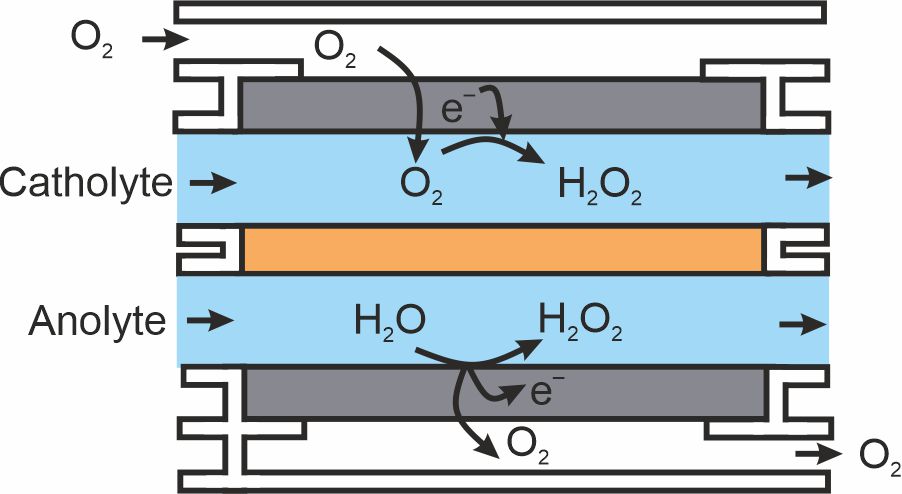
Figure 3. Schematic design for a hypothetical reactor where H2O2 is produced at the positive and negative electrode at the same time, giving a massive increase in production rate and energy efficiency. Figure reproduced with permission from Perry et al., copyright Elsevier B.V. 2021.
This is an excellent example of how the next great advancements in green chemical synthesis must come from a combined and unified approach, where multiple green technologies are combined in order to work towards a common goal.

