Authors: Simon S. Pedersen, Gabriel M. F. Batista, Martin L. Henriksen, Hans Christian J. Hammershøj, Kathrin H. Hopmann, and Troels Skrydstrup
Journal: JACS Au
Year: 2023
Featured image by jhenning from Pixabay
—
Poly(ethylene terephthalate), or PET, is one of the most commonly used plastics worldwide, with production in 2018 exceeding 30 million metric tons. In the textile industry, PET is better known as polyester – the same material used to make raincoats and athletic jerseys. It’s also a major component in plastic beverage bottles and food packaging, and it’s used to make Mylar for everything from survival blankets to party balloons.
Unfortunately, the majority of PET produced each year ends up in landfills or incinerated. In 2018, less than 30% of PET bottles produced that year were recycled due to unclear recycling guidelines and insufficient infrastructure. Since most PET today is produced from nonrenewable resources like crude oil, the low recycling rate means we likely won’t be able to keep up with global demand for PET in the future. Furthermore, relying on crude oil to make plastics contributes to climate change and adds CO2 to the atmosphere.
In the long run, ditching PET entirely and switching to reusable or refillable containers is the most environmentally friendly change we can make with regard to single-use plastics. But while we develop the infrastructure, we can mitigate the environmental impact of PET production by finding a more renewable way to make PET. For example, Coca-Cola’s PlantBottle program sources ethylene glycol, one of the precursors to PET, that is derived from plants rather than crude oil. Though the program is still developing, scientists are looking for more ways to make PET from renewable resources.
Researchers at Arhaus University in Denmark have found a way to synthesize methoxyterephthalic acid (MTA) from sawdust. MTA is a precursor to methoxy poly(ethylene terephthalate) (MPET), which is closely related to PET and can be used in similar applications. The team started from sawdust because it contains lignin, a natural polymer made of smaller units (called monomers) that have similar structure to MTA.
Figure 1 shows how the team synthesized MTA from sawdust. The researchers collected sawdust samples from eight different types of trees and broke down the lignin in each sawdust sample into monomers. From there, they swapped the hydroxide (OH) group on the monomers for a cyano (CN) group. This surprisingly complex reaction makes it easier to form the first carboxylic acid (COOH) group on MTA. The second carboxylic acid group was synthesized through the Amoco process, in which the monomer reacts with oxygen gas over cobalt, manganese, and bromide catalysts.
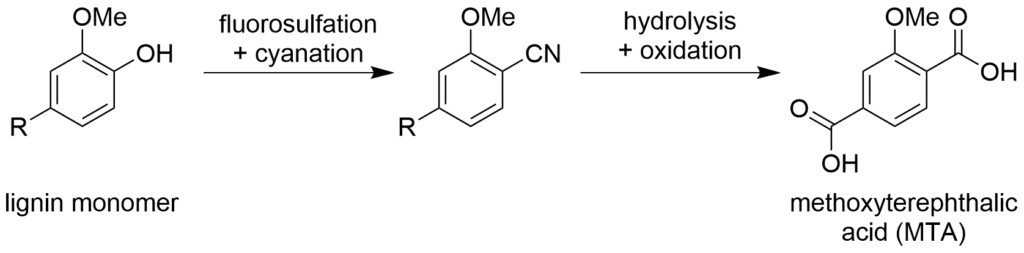
Figure 1. Synthesis of methoxyterephthalic acid (MTA). Adapted from Pedersen et al.
The team also compared the yields of the cyanated product formed just before MTA, depending on whether they used sawdust from hardwood or softwood trees. They found that they got the highest overall conversion of monomers from hardwood trees like birch or maple (98% average conversion from hardwoods vs. 30% from softwoods), but when they used softwood like spruce or noble fir, they eliminated unwanted side products. Since softwood trees generally grow faster than hardwood trees, both sources could be useful for making MTA at larger industrial scales.
Reducing the amount of PET that ends up in landfills requires long-term changes, including limiting production of single-use plastics and replacing single-use plastics with reusable containers whenever possible. In the meantime, though, we can turn to more sustainable ways of making PET, like using renewable resources as starting materials for PET synthesis. This paper offers one option for reducing our dependence on crude oil and moving towards biobased plastics.

