Authors: Xin Ren, Donggang Tao, Yudi Tang, Yuliang Cao and Fei Xu
Journal: Journal of Materials Chemistry A
Year: 2023
Image credit: Warut Roonguthai
As the world transitions towards renewable sources of energy for electricity production and transportation, there is a need for new storage technologies to hold energy that is produced. One storage technology that is being developed is magnesium batteries. Magnesium batteries take advantage of magnesium ions’ light weight and ability to store two electrons. Electrons in the battery move between a solid magnesium metal anode and a cathode that can hold magnesium ions in slots created by another chemical.
Often, these “slots” are created by crystal lattices. These crystal lattices, however, can be hard for magnesium ions to find available slots in, making the battery work slowly. A recent work by researchers at Wuhan University reported the use of a flexible polymer cathode in place of these crystal lattices to increase the amount of energy the battery could store.
The researchers’ design uses anthraquinone cores linked by sulfur bonds to create long polymers. By adding different amounts of sulfur, they created polymers that were linked by 1, 1.4, 1.9, and 2.2 sulfur atoms between each anthraquinone (Figure 1). These numbers are fractions because the number of sulfur atoms between each link in the polymer was not constant. For example, the 1.4 polymer has mostly bonds with 1 sulfur atom and some bonds with 2 atoms.
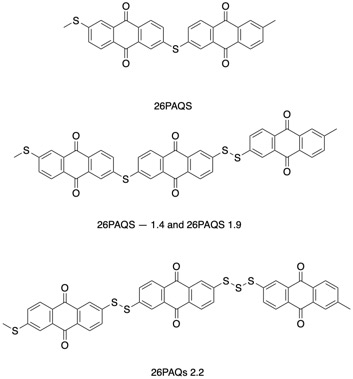
Figure 1: The anthraquinone cores were linked by sulfur bonds to form a polymer of many units. The 1.4 and 1.9 polymers differ by the percentage of single-sulfur connections and two-sulfur connections.
The researchers found that, as long as there were not more than 2 sulfurs bonded together to link the polymer, the stability of the polymer chains was strong. Additionally, they found that, when there was more than one sulfur, the battery could store more electrons, and consequently more energy, than would otherwise be expected. The researchers attribute this to the formation and disappearance of sulfur-oxygen double bonds as the battery is charged and discharged (Figure 2).
While magnesium batteries are not the primary battery technology used commercially today, they do hold promise in the future because of magnesium’s ability to carry a lot of charge in little mass. This research pushes forward our understanding of how magnesium batteries work and push them closer to being practical for widespread use.
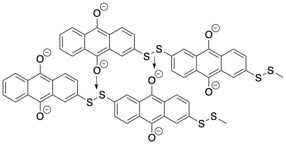
Figure 2: The polymer stores extra capacity by forming bonds between sulfur and oxygen.

