Title: Electroless deposition of tellurium nanowires in eutectic solvents using immobilised silver islands
Authors: Samuel Perry, Joshua White, Iris Nandhakumar.
Year: 2023
Journal: RSC Advances
Cover image credit: Marek Piwnicki
We are all aware of the need to move away from fossil fuels for our energy production. There have been great advancements in producing electricity form renewable energy sources such as sunlight (photoelectric) or heat (thermoelectric) generators. In many cases, we already have a strong idea of the sort of materials that are likely to give us the largest amount of electrical energy, which is essential if we want to completely remove fossil fuels from our national energy grids. In these cases, much of the research involves taking materials that are already pretty good, and making small modifications in order to make them even better.
Modifications such as these don’t get much smaller than nanostructures. A material is said to be nanostructured if it has features that can be measured in terms of tens of nanometers. Sizes this small are pretty hard to picture and even more challenging to make – a nanometer is one millionth of a millimeter, or about 1/10,000 of a human hair. Nanostructures themselves come in a wide variety of shapes and sizes, with researchers investigating the power of nano-wires, rods, ribbons, particles, and tubes to name just a few. Importantly, transforming a material into a nanostructure has been shown to greatly improve its performance for solar and thermal green energy production, so the hunt is on for new and exciting nanostructured materials.
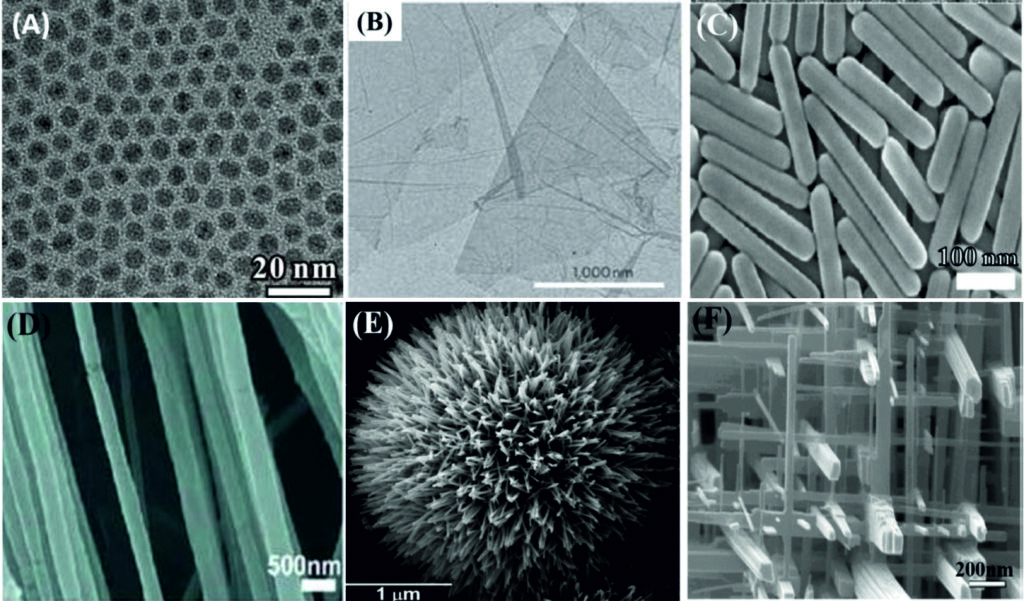
Making these structures usually requires some highly complicated experimental systems. In the case of nanowires, this usually involves taking a template with tiny nanometer-sized holes with the material, then trying to remove the template to leave the nanowires behind. This technique can produce some efficient nanowires, but comes with a couple of sizeable challenges. The pores can become blocked, which stops the wires growth, and it’s very easy to accidentally break the wires when trying to remove that template. There is therefore a great interest in finding new ways that are capable of producing these nanostructures.
Researchers at University of Southampton were facing this challenge in trying to make nanowires out of tellurium, a material that can efficiently convert heat into electrical energy. Their plan was to use electrodeposition, where negative electrical energy is passed through gold electrode in a solution containing positively charged tellurium ions. The negative potential would convert the tellurium ions that come into contact with the gold into a solid tellurium layer. To their surprise, when the gold electrode was dipped into the tellurium solution, a black film started to spontaneously grow. Even more surprising, when they investigated this black layer, they were even more surprised to find a layer of pristine tellurium nanowires, despite using no energy to make the film and adding no template to try and make nanowires!
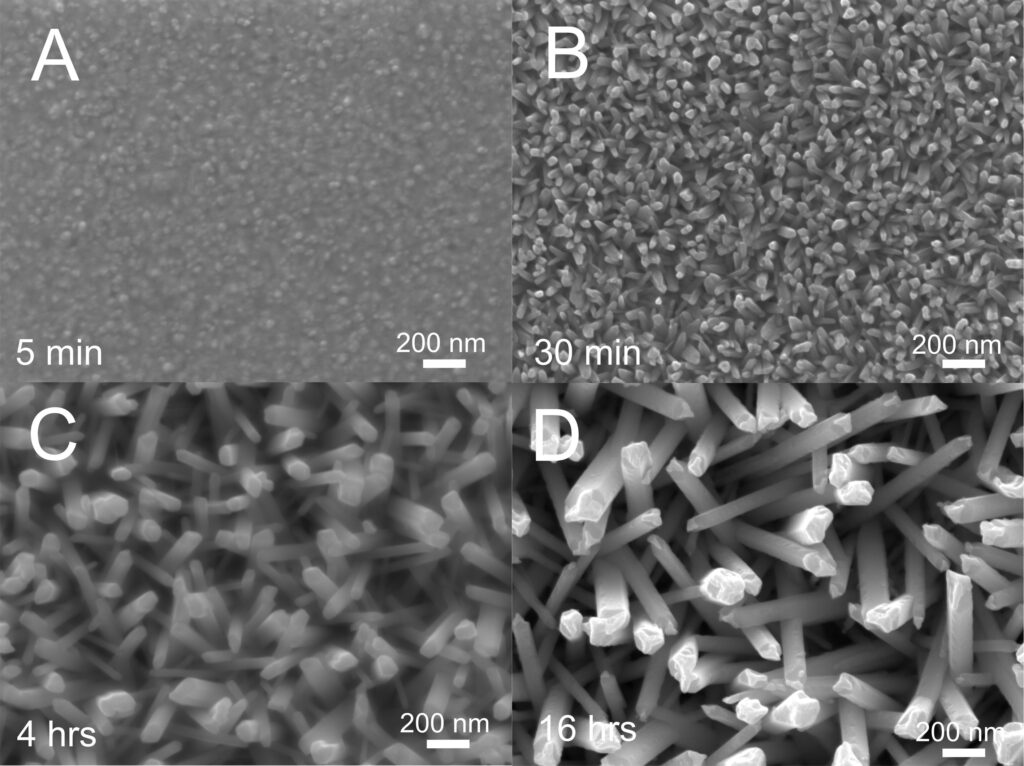
It turns out the electrode was experiencing a phenomenon known as ‘electroless deposition’ caused by a small piece of exposed silver that was on the surface of the gold electrode. Silver is more oxidising than tellurium, and so when the silver is immersed in the tellurium solution, the silver spontaneously dissolves and the tellurium spontaneously deposits without needing any external energy input. This gives the impression of tellurium depositing on its own without having to do anything to cause it!
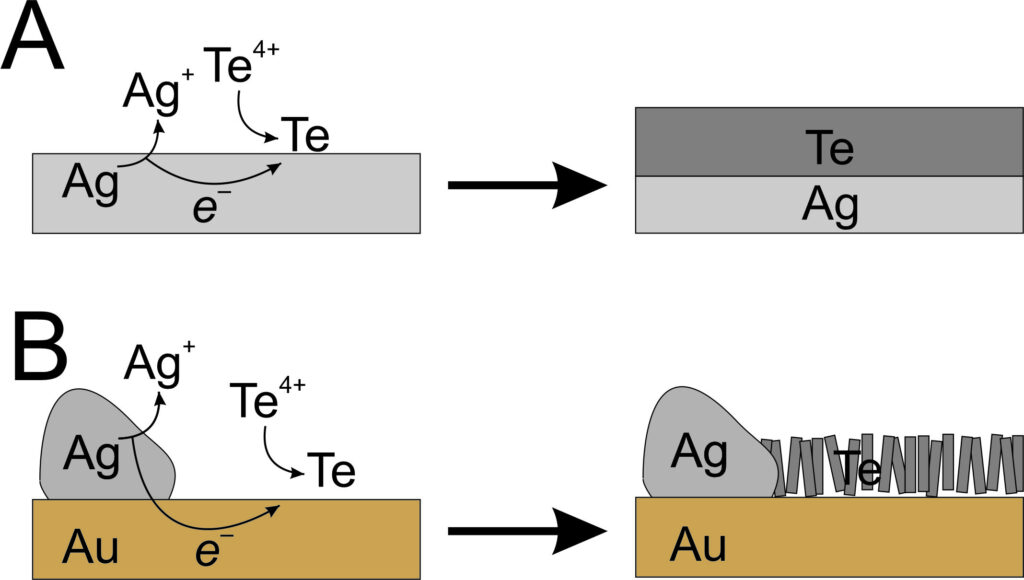
Normally, electroless deposition causes a material to deposit on the same substance that is dissolving – in this case this would be tellurium depositing onto a piece of silver metal. In this case, a relatively smooth tellurium film would be seen, rather than the desired array of nanowires. What is distinct about this case is that the silver is isolated on the corner of a piece of gold, and the deposition is occurring at a different site on the smooth gold surface. The silver is electrically connected to the gold, which means it is now possible for silver to dissolve at one site and tellurium to deposit at another. This is similar to the technique of galvanic protection where more oxidising zinc is bolted onto steel surfaces. The zinc preferentially dissolves which helps to slow the rate of rusting in ship hulls and underwater pipes.
Since the deposition is controlled by how quickly the silver can dissolve, and the gold surface has a particular surface The electroless deposition of tellurium is controlled by how quickly the silver can dissolve, which is relatively slow. This may seem like a disadvantage, but since the gold surface has a particular surface structure that aligns well with tellurium nanowires, the slow deposition rate allows the tellurium to steadily grow in the desired nanowire shape. This offers a new way to make an array of nanowires out of a highly useful material without needing any energy input or complicated templates.

