Title: Catalytic Fabric Recycling: Glycolysis of Blended PET with Carbon Dioxide and Ammonia
Authors: Yang Yang, Shriaya Sharma, Carlo Di Bernardo, Elisa Rossi, Rodrigo Lima, Fadhil S. Kamounah, Margarita Poderyte, Kasper Enemark-Rasmussen, Gianluca Ciancaleoni, and Ji-Woong Lee
Journal: ACS Sustainable Chemistry & Engineering
Year: 2023
Header image adapted from Yang et al.
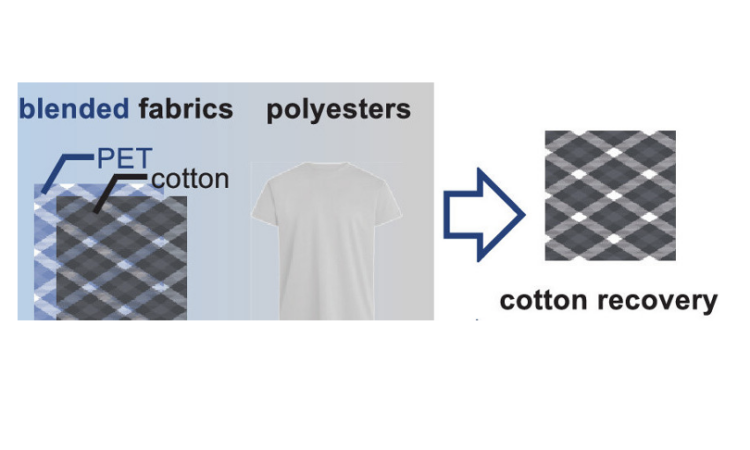
Many fabrics consist of a blend of polyester and cotton. In order to recycle these textiles, the cotton and polyester must first be separated. However, the intertwined nature of these fabrics makes it incredibly challenging to separate these materials. As a result, most textile is either placed in a landfill or incinerated. Now, thanks to researchers from the University of Copenhagen, recycling fabrics may someday become a reality.
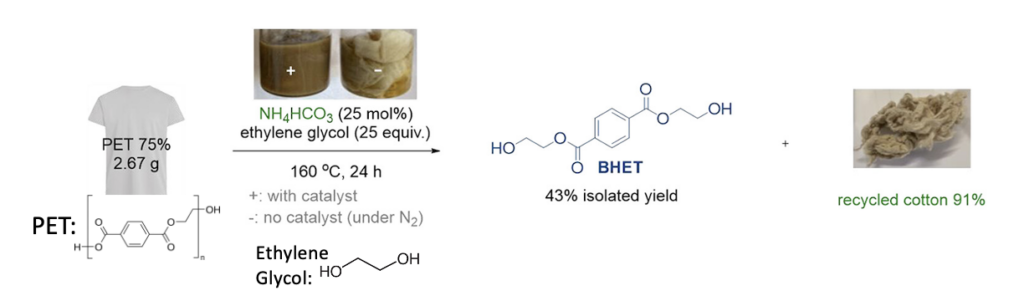
Yang et al. recently developed an efficient and scalable way to recycle blended fabrics by chemically breaking down the polyester into its recyclable monomeric units, called BHET, which can be re-polymerized into polyester. While doing so, the chemical reaction leaves the cotton intact. This is difficult to do since cotton contains labile bonds that are susceptible to degradation under many conditions. Through a rigorous screening and optimization of reaction conditions, they found that the ordinary household ingredient of Hartshorn salt or baker’s ammonia (ammonium bicarbonate, NH4HCO3) can serve as a precatalyst to selectively break down polyester, leaving the cotton intact. This transesterification process involves heating the blended fabric with ethylene glycol and a catalytic amount of NH4HCO3 at 160 °C (Figure 1). This one-pot reaction is metal-free, cost-effective, safe, and easy to carry out! In the example below, 43% of the BHET monomer was isolated with high purity and 91% of the cotton was recovered from an ordinary t-shirt.
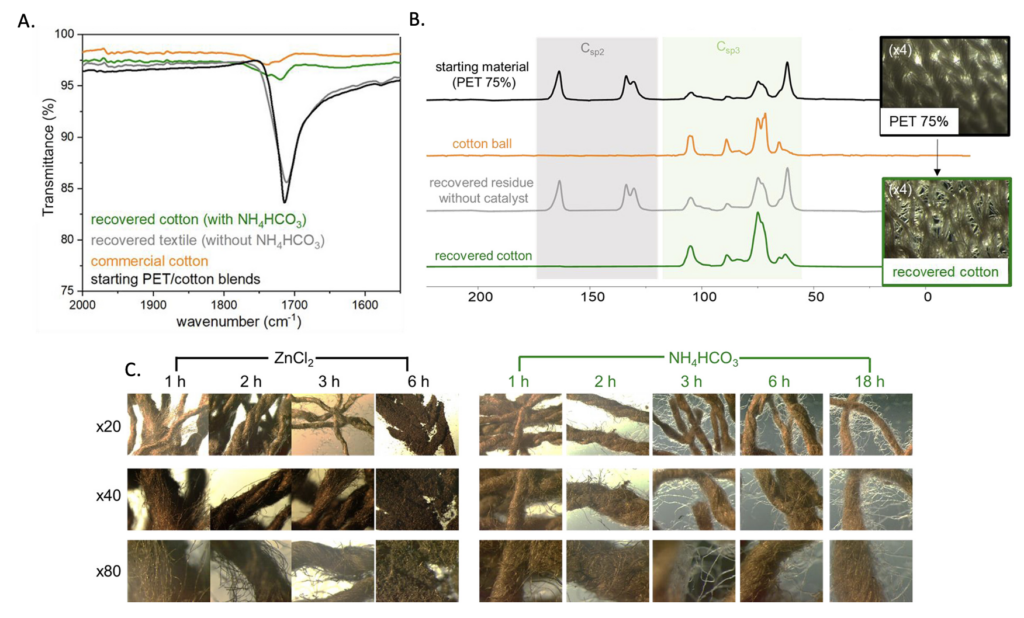
To get a better look at the quality of the recovered cotton, the researchers employed a variety of analytical techniques including IR, NMR and optical microscopy. Figures 2A and 2B show the IR and NMR data of commercial cotton (orange) compared to the cotton recovered from the blended fabric using their reaction (green). The orange and green traces are similar, suggesting that the recovered cotton is intact. This is further verified with microscopic images which show the cotton over the course of the reaction (Figure 2C). This is compared to a reaction using ZnCl2 as the catalyst – which is known to degrade cotton – instead of NH4HCO3. The difference between the two sets of images is striking. By 6 hours, the cotton threads are disintegrated when using ZnCl2. However, the strands remain intact when using NH4HCO3.
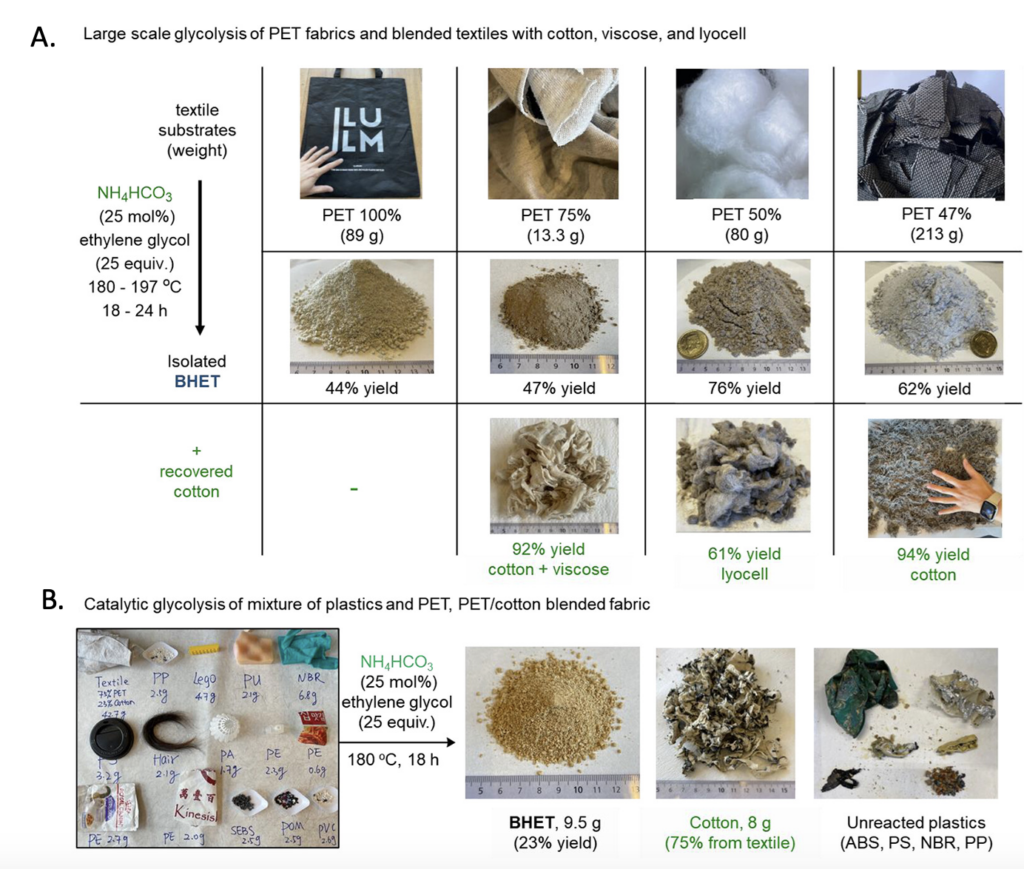
To demonstrate the vast applicability of this reaction, the researchers subjected various blended fabric materials to their reaction in separate pots. These items included a grocery bag, t-shirt, quilt filling, and a sofa cover (Figure 3A). In all cases, the reaction worked! The cotton yields range from moderate to excellent. In addition, to better illustrate how the reaction would perform on realistic waste, the group subjected a heterogenous mixture of items to their reaction including textile, a lego, human hair, a nitrile glove, and wrappers. They found that they were still able to recover 75% of the cotton and isolate 23% of the BHET polyester precursor (Figure 3B).
Overall, the researchers developed a reaction that allows for polyester recycling and cotton recovery from blended fabrics. This can be applied to an array of different household items and even complex waste mixtures. Hopefully, green recycling strategies like this one can become mainstream to allow for a more sustainable production of textiles and circular economy.
~~~
This is an unofficial adaptation of an article that appeared in an ACS Sustainable Chemistry & Engineering publication. The ACS has not endorsed the content of this adaptation or the context of its use.
Figures are adapted with permission from Yang, Y.; Sharma, S.; Di Bernardo, C.; Rossi, E.; Lima, R.; Kamounah, F. S.; Poderyte, M.; Enemark-Rasmussen, K.; Ciancaleoni, G.; Lee, J.-W. Catalytic Fabric Recycling: Glycolysis of Blended Pet with Carbon Dioxide and Ammonia. ACS Sustainable Chemistry & Engineering 2023, 11 (30), 11294–11304. Copyright 2023 American Chemical Society.
Full Reference: Yang, Y.; Sharma, S.; Di Bernardo, C.; Rossi, E.; Lima, R.; Kamounah, F. S.; Poderyte, M.; Enemark-Rasmussen, K.; Ciancaleoni, G.; Lee, J.-W. Catalytic Fabric Recycling: Glycolysis of Blended Pet with Carbon Dioxide and Ammonia. ACS Sustainable Chemistry & Engineering 2023, 11 (30), 11294–11304. DOI:10.1021/acssuschemeng.3c03114.