Featured image reprinted with permission from Wang, M. et al, ACS Energy Letters 2020, 5, 758-765. Copyright 2020 American Chemical Society.
Paper: Powering Electronics by Scavenging Energy from External Metals
Authors: Min Wang, Unnati Joshi, and James H. Pikul
Modern robots, from Roombas to Mars rovers, are revolutionizing modern life. But robot design can be limited by the need to include a power source for the robot. For very small robots, technologies like lithium ion batteries can become prohibitively heavy while only providing a short duration of power. To overcome the limitations of traditional energy storage in small robots, the authors of a recent paper in ACS Energy Letters demonstrate a novel strategy that scavenges energy from a metal surface to power a small vehicle.
Most robots rely on technologies like lithium ion batteries to store energy. Lithium ion batteries can provide high energy densities, which is great for robots that might contain electronics drawing a large amount of power. However, smaller lithium ion batteries weigh more than large lithium ion batteries relative to the total amount of energy they can provide, making them impractical for small robots.
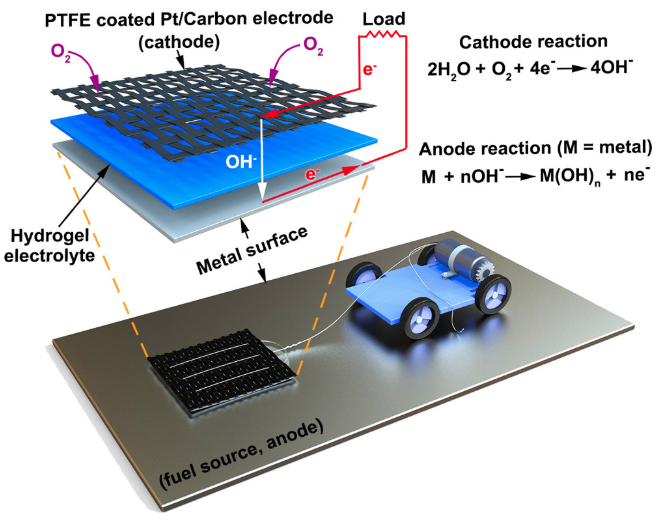
Instead, the authors propose a metal-air scavenger, which consists of a cathode (electrode where reduction occurs) that can produce hydroxide ions and a metal anode (electrode where oxidation occurs), which can be oxidized by the hydroxide ions. As the device travels across a metal surface, the hydroxide ions produced by the cathode can oxidize the metal surface, providing the robot with electricity. Attached to the cathode is an electrolyte made of a hydrogel, which consists of a 3D network of long polymer chains that can absorb and store water. The electrolyte connects the cathode towed by the robot to the metal surface serving as the anode.
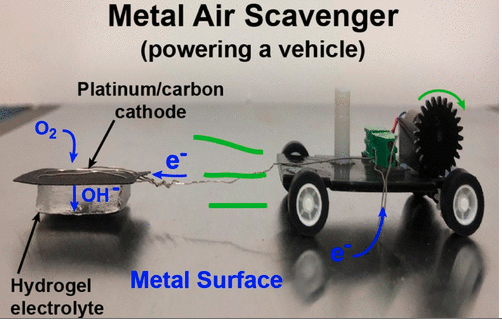
The advantage of the metal-air scavenger system is that it allows the robot to extract energy from anywhere on the metal surface without having to carry a source of energy on board the robot. The authors demonstrate that the metal-air scavenger can generate relatively high power densities on aluminum and zinc surfaces and lower power densities on stainless-steel surfaces. As the amount of current generated by the device relies on the oxidation of the metal, the device is more or less efficient depending on the metal’s reduction potential. Although oxidation of the metal by the electrode reduces the amount of energy available to the robot on a second pass, the authors report that the metal-air scavenger can still extract energy from oxidized metal surfaces.
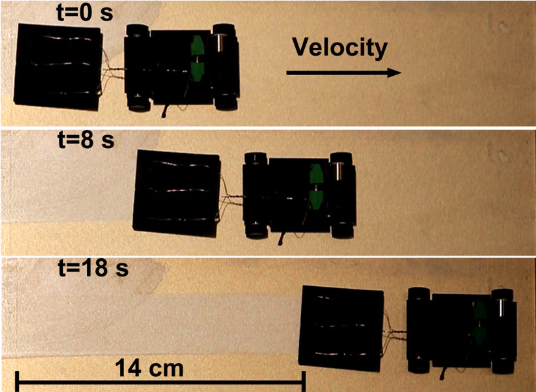
The type of hydrogel used as an electrolyte in the metal-air scavenger can also affect device performance. The authors studied the oxidized metal surfaces after the robot had passed over them and found that when the the less deformable PMA hydrogel was used, the surfaces were rougher and device performance was lower than when PVA hydrogel was used. As the less-elastic PVA does not penetrate into the metal oxide as it forms, it maintains a more consistent current density, resulting in improved device performance.
While metal-air scavengers may not replace Roombas, the high performance and efficiency of the metal-air scavengers created by the authors demonstrate how useful this energy scavenging method could be. Small robots that inspect metal surfaces or operate in areas with scrap metal could benefit from the low weight and micro-sized scale of devices like the metal-air scavenger, allowing robots to perform even more useful tasks in modern life.

